Ligands/Coordination compounds (Openstax Ch. 19)
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Complex ion
Consist of metal cations that form coordinate covalent bonds with ligands
Ligand
Molecules that have at least one e- pair that it can share in a LAB interaction
Coordination number
Gives the total number of e- pairs shared with the metal cation
Coordination compound
Formed when complex ions are combined with 1 or more counterions
Monodentate ligand
Share ONE e- pair with the metal cation (ex. NH3, Cl-, CN-)
Bidentate ligand
Share TWO e- pairs with the metal cation (ex. C2O42-, H2NCH2CH2NH2 (en))
Polydentate ligand
Share MORE THAN TWO e- pairs with the metal cation (ex. EDTA)
Octahedral geometry coordination number
6
Tetrahedral geometry coordination number
4
Square planar geometry coordination number
4
Isomer of coordination compounds
Compounds with the same formula, but different structures
Structural isomers
Different connectivity
Coordination sphere isomer (type of structural isomer)
When the ligands exchange places with uncoordinated counter ions
linkage isomers (type of structural isomer)
Contain ligands that coordinate to the metal cation in different ways (NO2-, CN-, OCN-, SCN-)
Stereoisomers
Have the SAME connectivity but different spatial arrangements
Geometric isomer (type of stereoisomer)
Have ligands in different spatial arrangements around the metal cations
Cis geometric isomer
The two atoms are on the same side (90 degrees)
Trans geometric isomer
The two atoms are on opposite sides (180 degrees)
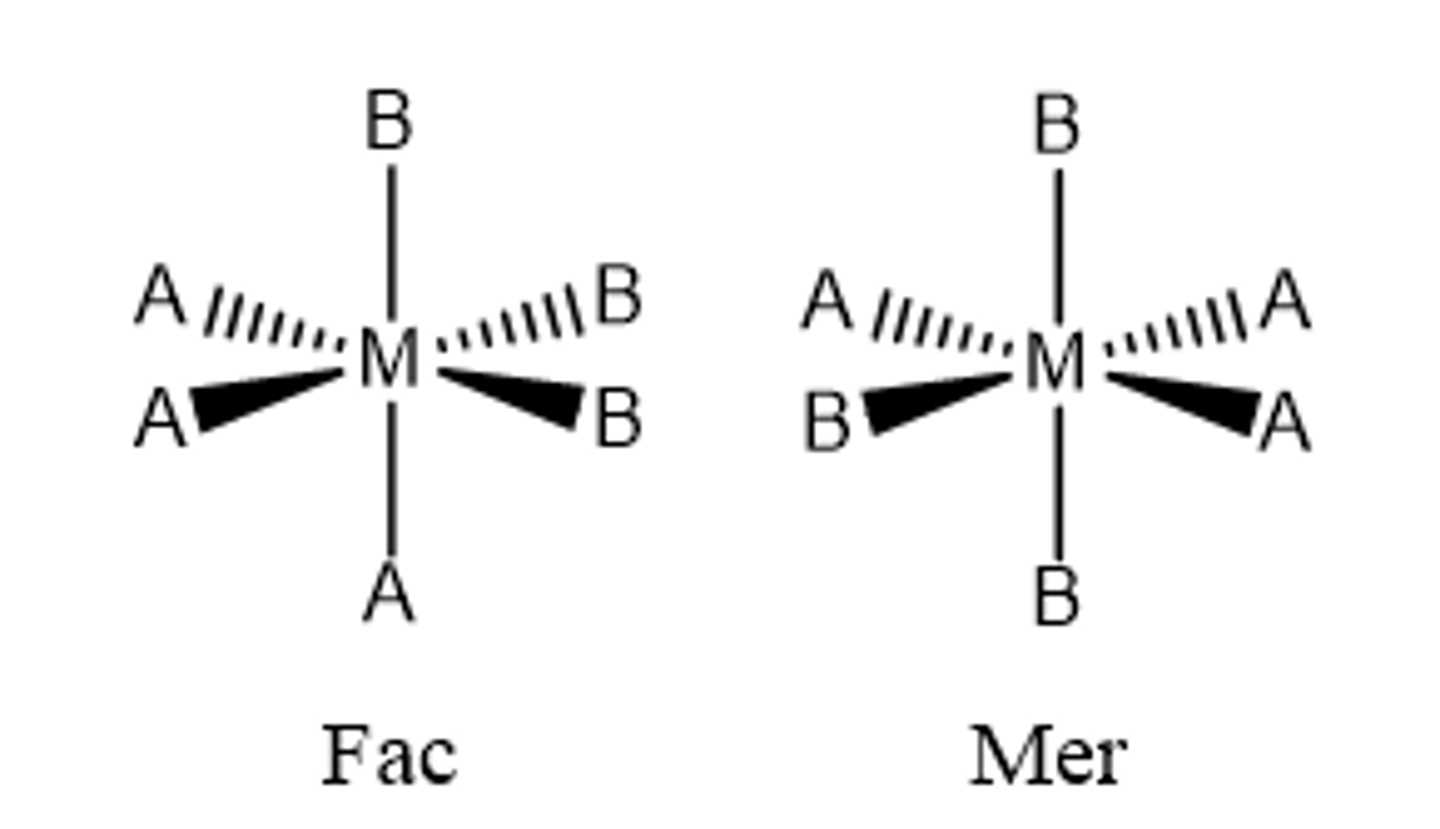
fac geometric isomer
Any two identical ligands are adjacent to one another (cis)
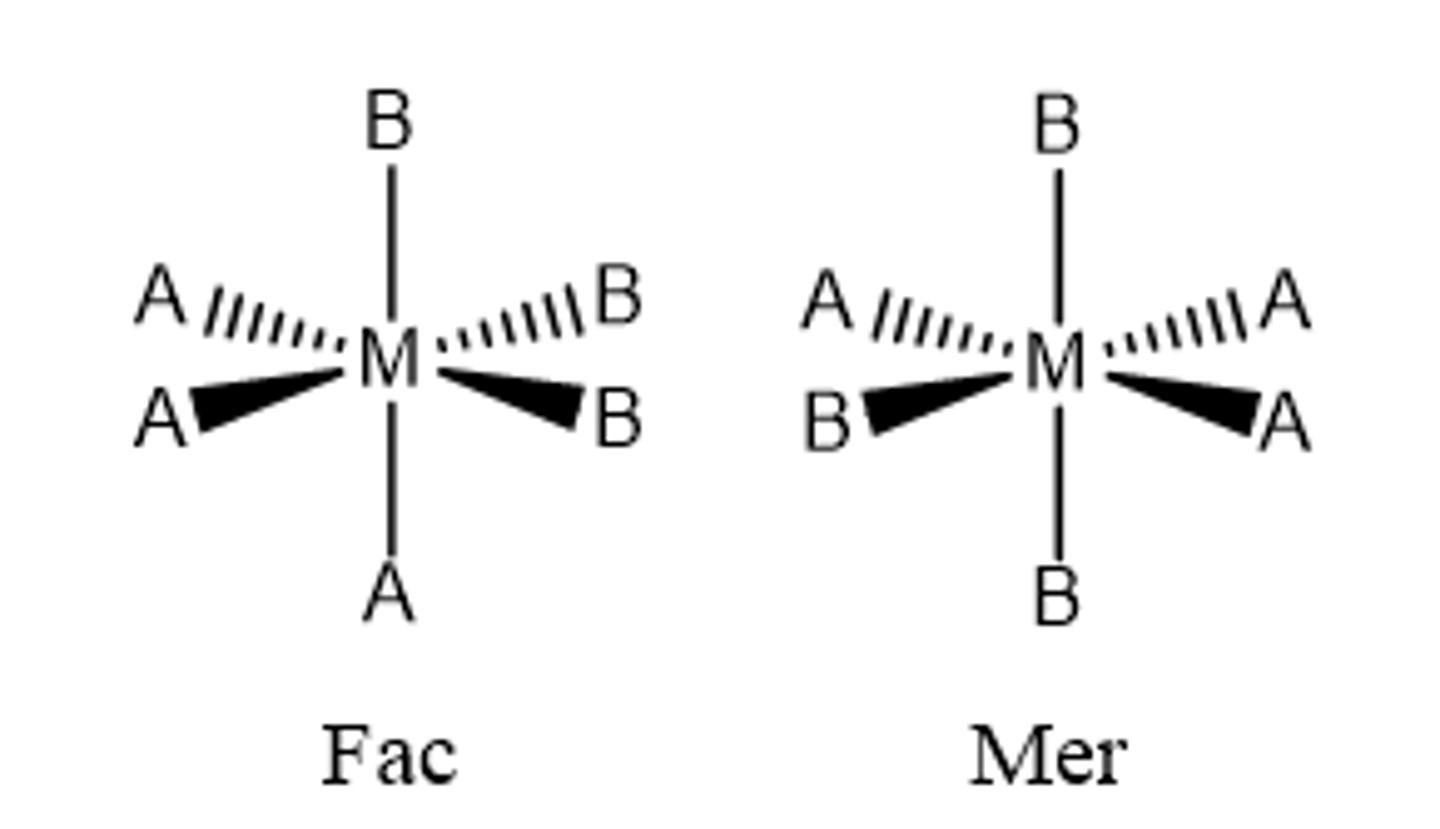
mer geometric isomer
A combination of a cis/trans isomer
Optical isomers
Mirror image of one another
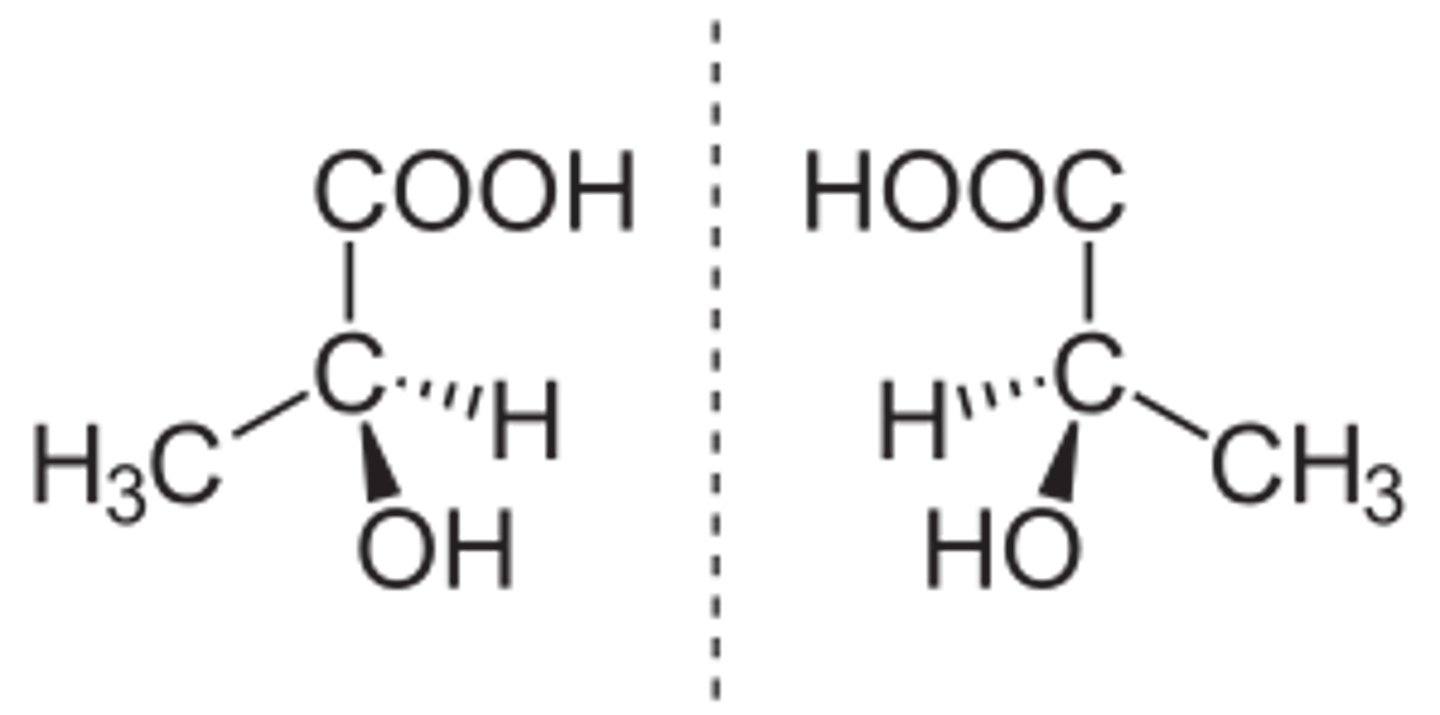
Crystal Field Theory for Octahedral Complexes
When a metal cation is in an octahedral electric field created by the ligands, the d orbitals will INCREASE in energy
Crystal Field energy
The difference in energy between the two groups of d orbitals
Small crystal field energy
absorb long wavelengths, reflect short wavelengths; high spin (crystal field energy < e pairing energy), orbitals not filled
Large crystal field energy
absorb short wavelengths, reflect long wavelengths; low spin (crystal field energy > e pairing energy), orbitals all filled