Edexcel iGCSE (9-1) Chemistry | Solubility Rules
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Solubility rules
• All sodium, potassium and ammonium compounds are soluble.
• All nitrates are soluble.
• All chlorides are soluble except for silver and lead(II).
• All sulfates are soluble except for barium, calcium and lead(II).
• All carbonates and hydroxides are insoluble, except for those of sodium, potassium and ammonium. Calcium hydroxide is slightly
soluble.
Is Na2O soluble?
Yes - all sodium compounds are soluble
Is KCl soluble?
Yes - all potassium compounds are soluble
Is NH4Br soluble?
Yes - all ammonium compounds are soluble
Is LiNO3 soluble?
Yes - all nitrates are soluble
Is RbCl soluble?
Yes - all chlorides are soluble except those of silver and lead (||)
Is AgCl soluble?
No - all chlorides are soluble except those of silver and lead (||)
Is PbCl2 soluble?
No - all chlorides are soluble except those of silver and lead (||)
Define a solvent
The substance that dissolves a solute
Define a solute
The substance that is dissolved by a solvent
Define a solution
A mixture of a solute and a solvent which is the same throughout
Define a saturated solution
A solution where the maximum amount of solute has been dissolved, so no more solute will dissolve
Define a supersaturated solution
A substance heated up to a greater temperature then become saturated, then cooled, meaning that the solution has more solvent than it could have dissolved a that temperature - a solid forms
What increases solubility?
Temperature
What is a graph of solubility vs temperature known as?
A solubility curve
How is solubility measured?
The grams of solute that will dissolve per 100g of solvent
Define solubility
The ability of a substance to dissolve in a solvent
Draw an example solubility curve
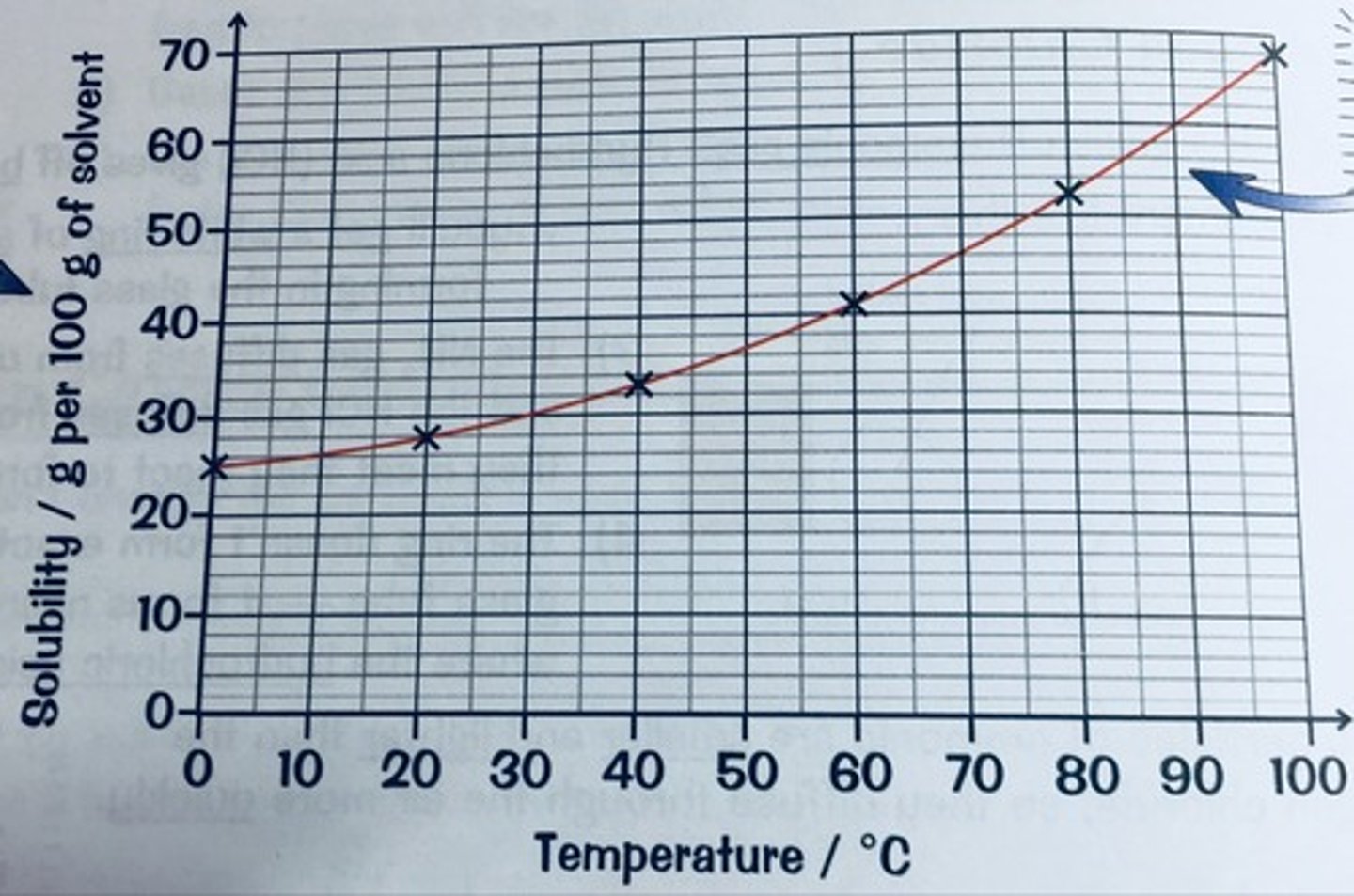
Describe an experiment to investigate how solubility is affected by temperature

Solubility equation
Solubility = mass of solid x 100 / mass of water removed