Unit 4: Acids and Bases (Part 2)
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Titration
Quantitative chemical analysis is used to determine the unknown concentration of a known reactant.
Equivalence point: stoichiometric equivalent amounts of reactants.
moles H+ = moles OH-Chemical indicators are used to help us determine this point.
Often, when titrations are done, several trials are necessary to check the accuracy.
Ideally, volumes should agree within ± 0.02 mL.
Only volumes within ± 0.02 mL are used to determine the average volume of titrant
Volumes that exceed this tolerance are usually discarded.
Five parameters of all titration
concentration of acid
concentration of base
acid/base ratio (almost always given)
volume of acid
volume of base

Percentage purity
Titrations are often used to calculate the percentage purity of a solid acid or base.
Actual concentration can be calculated from titration data.
Expected concentration can be calculated from the impure compound.
Titrations can also be used to calculate the molar mass of an unknown solid acid or base, provided the acid or base is known to be monoprotic, diprotic, etc.
Titration data allows us to calculate the moles of the acid or base.
Molar mass is given by dividing the mass og the acid or base used to prepare the solution by moles.
Indicator
A weak organic acid or base that has different colours for its conjugate acid and base form.
Indicators are often indicated by the symbol HIn (acid form) and In- (base form).
When an indicator is placed into an acidic solution, the excess H3O+ causes a shift in the indicator equilibrium.
HIn (yellow) + H2O ⇌ In- (red) + H3O+↑
→ An indicator will be its CONJUGATE ACID (HIn) form in HIGHLY ACIDIC solutions → [HIn] > [In-]
When an indicator is placed into a basic solution, the OH- reacts to decrease the [H3O+] and causes a shift in the indicator equilibrium.
HIn (yellow) + H2O ⇌ In- (red) + H3O+↑
→ An indicator will be its CONJUGATE BASE (In-) form in HIGHLY BASIC solutions → [HIn] < [In-]
Transition point
The indicator is midway through its colour change.
[HIn] < [In-]
KIn = [H3O+]
pKIn = pH
Standard solution (Standardized solution)
Solution with precisely known concentration.
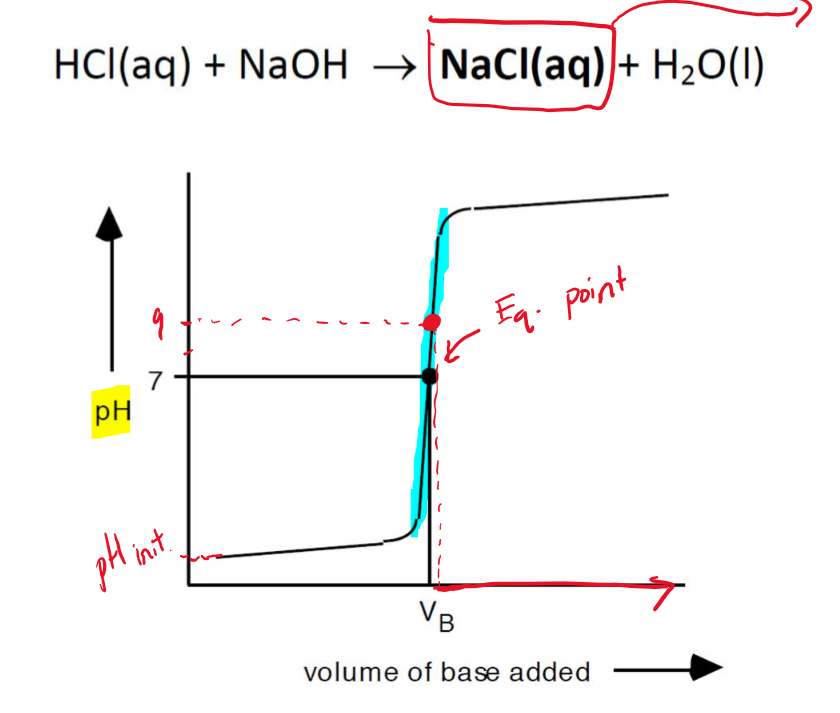
Titration of a STRONG ACID with a STRONG BASE
The pH rises almost vertically around the value of VB.
VB is the volume of the base required to reach the equivalence point.
STRONG ACID - STRONG BASE titrations produce NEUTRAL salt solution so pH = 7 at the equivalence point.
Choose the indicator which changes colour around pH = 7 (pKIn = 7), such as phenol red, neutral red, or bromthymol blue.
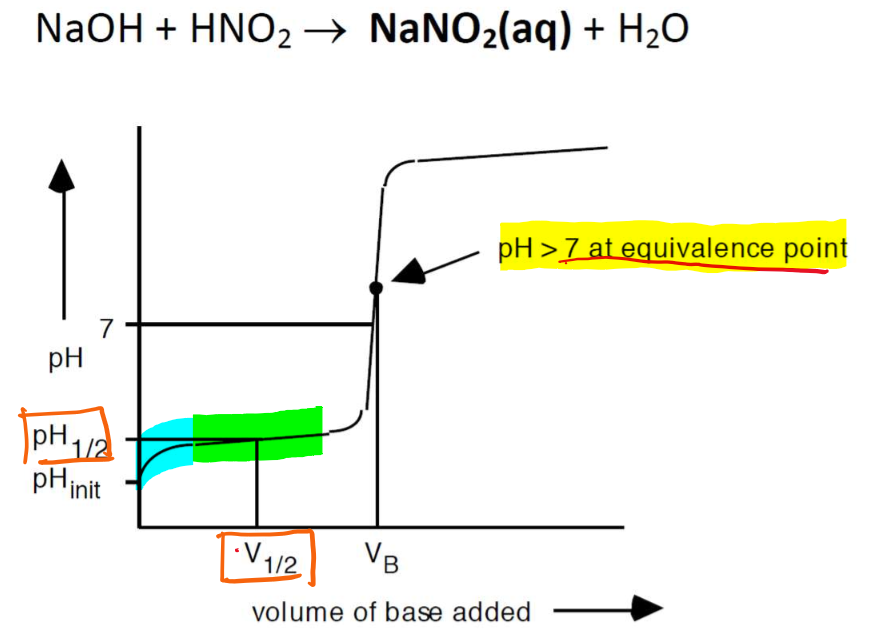
Titration of a WEAK ACID with a STRONG BASE
BUFFERING ZONE: Initial upswing in the pH at the start of the titration, and then a slowdown in pH change.
weak acid - STRONG BASE titration produces a BASIC salt solution. As such, the pH > 7 at the equivalence point.
(NaNO2 hydrolyzes to give a base)
NO2-(aq) + H2O(l) → HNO2(aq) + OH-(aq)Titration requires an indicator that changes colour at pH > 7 (pKa > 7), such as phenophthalein.
pH1/2 is the midpoint of the titration, at which half of the weak acid has been neutralized (i.e., halfway point for the titration)
pKa of the weak acid can be determined at this pH.
[HNO2] = [NO2-]
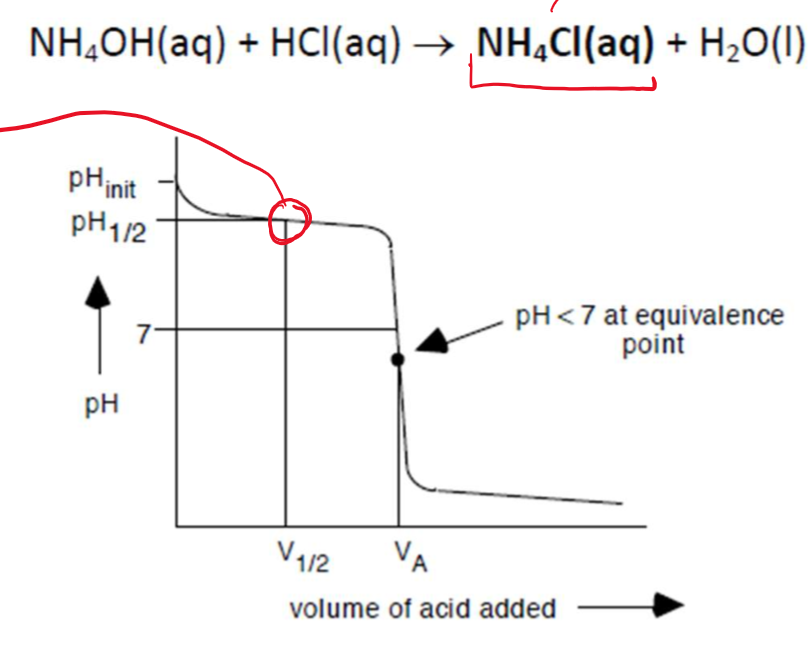
Titration of a weak base with a STRONG ACID
Similar to weak acid and strong base, except that the curve is flipped upside down.
The pKb of the weak base can also be determined from the midpoint, but remember that the graph gives pH values, not pOH; therefore, the pH needs to be converted to pOH.
weak base - STRONG ACID titration produces an ACIDIC salt solution. As such, the pH < 7 at the equivalence point.
(NH4Cl hydrolyzes to give acid)
NH4+(aq) + H2O(l) → NH3(aq) + H3O+(aq)The titration requires an indicator that changes colour at pH < 7 (pKa < 7), such as methyl red.
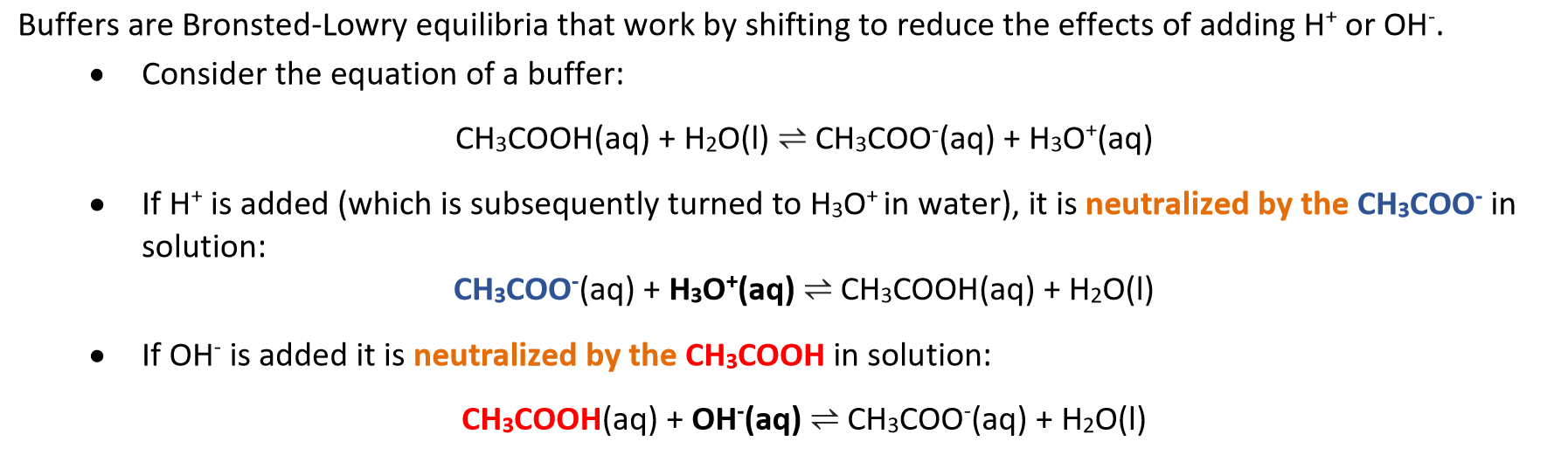
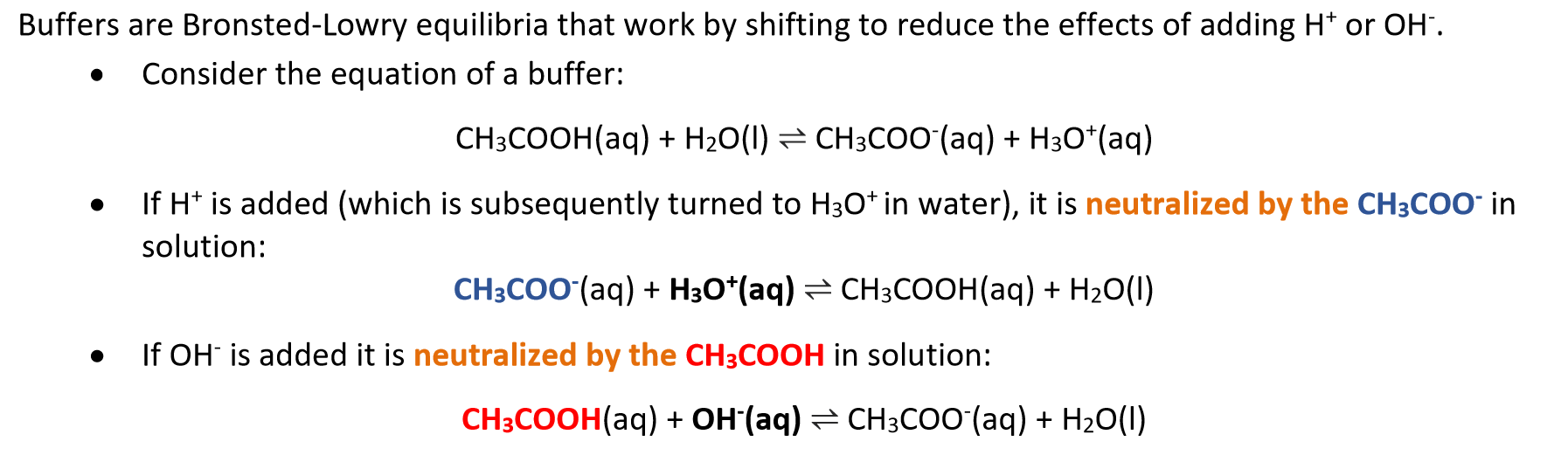
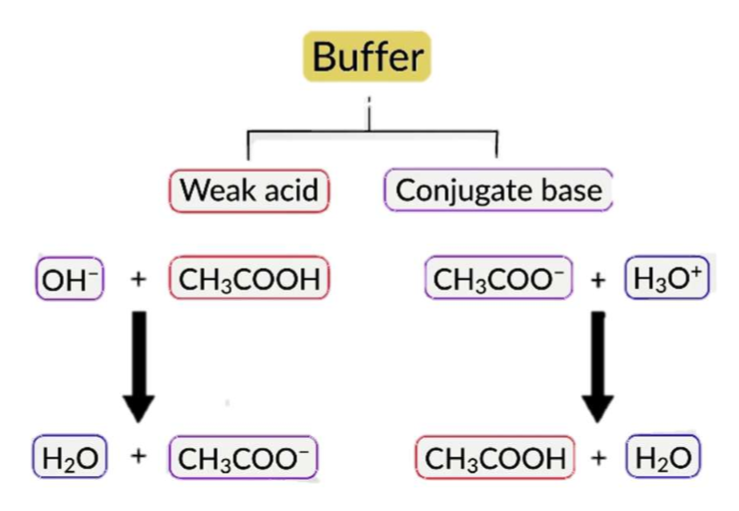
Buffer
Solution where the solutes protect it against large changes in pH, even when strong acids or bases are added.
consists of a weak acid and its conjugate weak base in roughly equal concentration, or vice versa.
Often added as soluble salts
CH3COOH and NaCH3COO (acidic buffer - pH < 7)
NH4NO3 and NH3 (basic buffer - pH > 7)
NaH2PO4 and Na2HPO4
Bronsted-Lowry acids and bases do not act effectively as buffers on their own. Both conjugate acid and base must be present in adequate amounts to allow shifting in both directions.
Buffers are most effective when the pKa ≈ pH of the acid.
Buffer capacity
The amount, moles, of acid or base a buffer can react with until a large change in pH will occur.
The actual number of moles of acid and base that are used to prepare the buffer determines its capacity.
NOTE:
Diluting a buffer has no effect on its buffer capacity because the capacity is dependent on the moles.
Diluting a buffer has no effect on its buffer capacity because both conjugate acid and base are diluted equally.
Buffers DO NOT maintain a CONSTANT pH; they simply resist large changes.
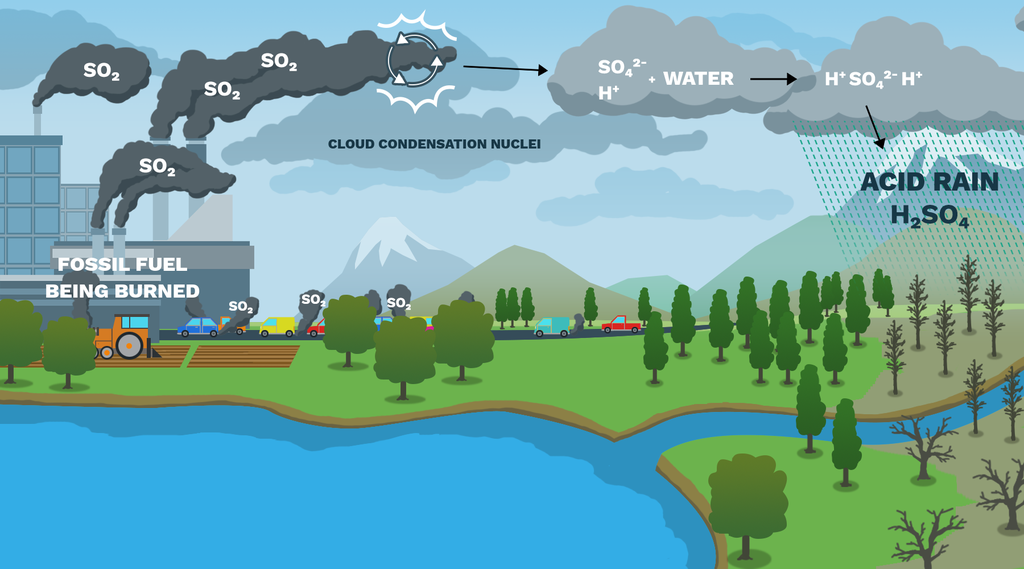
Acid rain
Rain is naturally acidic due to dissolved CO2 in the atmosphere giving it a pH of about 5.6.
Any precipitation that has a pH < 5.56 is called ACID RAIN.
Sources of acid rain
All fossil fuels, like coal and oil, can contain sulphur, which reacts with oxygen when burned to form sulphur dioxide:
S + O2 ⇌ SO2
2SO2 + O2 ⇌ 2SO3
SO2 + H2O → H2SO3 (sulphurous acid)
SO3 + H2O → H2SO4 (sulphuric acid)
Combustion reactions in automobiles cause small amounts of N2 to react with oxygen in the air.
N2 + O2 ⇌ 2NO
N2 + 2O2 ⇌ 2NO2
2NO + O2 ⇌ 2NO2
2NO2 + H2O ⇌ HNO3 + HNO2 (nitric & nitrous acid)
→ This mixture of H2SO4, H2SO3, HNO3, and HNO2 all constitute “acid rain”. These compounds are naturally occurring (e.g., from volcanoes and rotting vegetation), but humans contribute a large portion of them.
Issues associated with acid rain
Affects fish and plant growth
Soil acidification
Loss of algae (food for fish or for organisms that fish eat)
Leaches minerals out of rocks and soils.
Poisonous substances from rocks, such as aluminum ions
Beneficial nutrients from topsoil
Erosion of limestone structure and buildings
Contamination of (clean) drinking water
Destruction of crops in agriculture