2. Bonding, Structure and The Properties of Matter
1/154
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
155 Terms
Solid to Liquid
Liquid to Solid
Liquid to Gas
Gas to Liquid
Solid to Gas
Gas to Solid
Melting
Freezing
Evaporation
Condensation
Sublimation
Deposition
Changes of state are..
physical changes because no new
substances are formed
Solids
Particles have low energy
Particles held in fixed position by forces
Particles vibrate but cannot move freely so solids keep their own shape / do not flow
Particles are close together so solids cannot be compressed
Liquids
Particles have more energy and move quite fast
Particles move freely so liquids flow
Particles are close together so liquids cannot be compressed
Gases
Particles have high energy so move very randomly
Very weak forces between particles so particles are far apart.
This means:
-gases can be compressed
-gases expand in all directions to fill their containers
Limitations of Particle Model
Particles are shown as solid spheres
It does not show weak forces between particles
It does not show movement/speed of particles
Bulk Properties
‘Bulk’ means the whole substance (all particles together)
Melting & boiling points and density are ‘bulk properties’ because they depend upon how all particles behave together
Ionic bonding
-attraction between oppositely charged ions
-occurs between metals and non-metals (name or formula of a compound has metal & non-metal = ionic)
Ionic bonding occurs because
metal atoms lose outer electrons to form + ions
non-metal atoms gain outer electrons to form - ions
the oppositely charged ions strongly attract each other
Ionic Charges - group:
1, 2, 3, 5, 6, 7
+1, Transfer/lose 1 electron
+2, Transfer/lose 2 electron
+3, Transfer/lose 3 electron
-3, Accept/gain 3 electrons
-2, Accept/gain 2 electrons
-1, Accept/gain 1 electrons
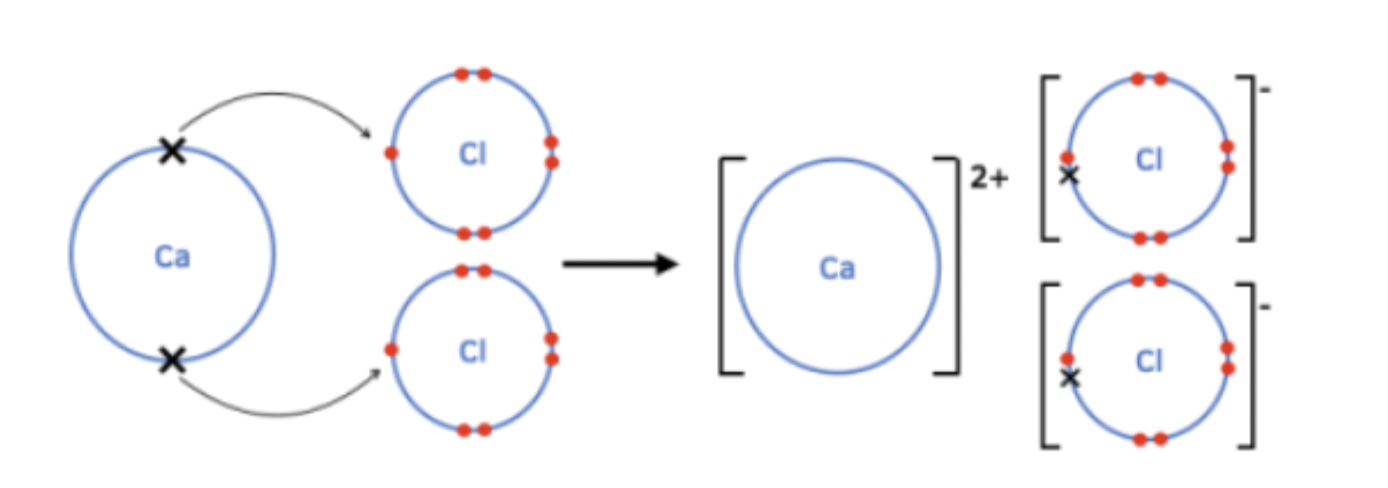
Example of ionic bonding question
Ca transfers 2 outer e to form Ca 2+
2 Cl each gain 1 e to form Cl -
Ca 2+ & Cl - ions strongly attract each other
Zn is…
+2
To work out the state (solid, liquid or gas) at room temperature,
compare room temperature
with each mpt & bpt:
-if room temperature is below mpt AND bpt: it has not even melted = solid
-if room temperature is between mpt & bpt: it has melted but not yet boiled = liquid
-if room temperature is above mpt AND bpt: it has melted and boiled = gas
Limitations of Ionic Models
2D Model: It only shows 1 layer of ions/does not show where the
other ions are
3-D model: It is not to scale/large gaps between ions
Dot & cross diagrams: Do not show the structure of the compound/how the ions are arranged
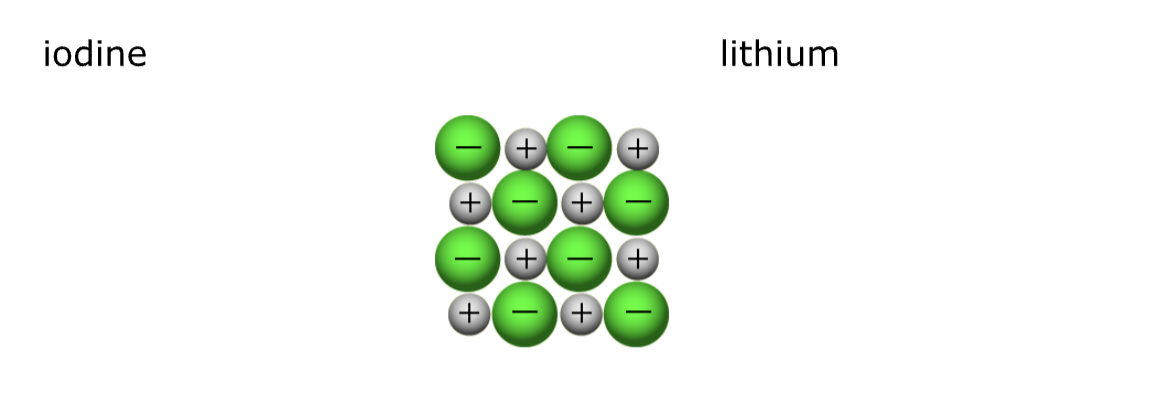
Predict Ionic formulae
This diagram has the same number of grey + (Li) & green – (iodine, I)
For every 1 x Li there is 1 x I
The formula is LiI
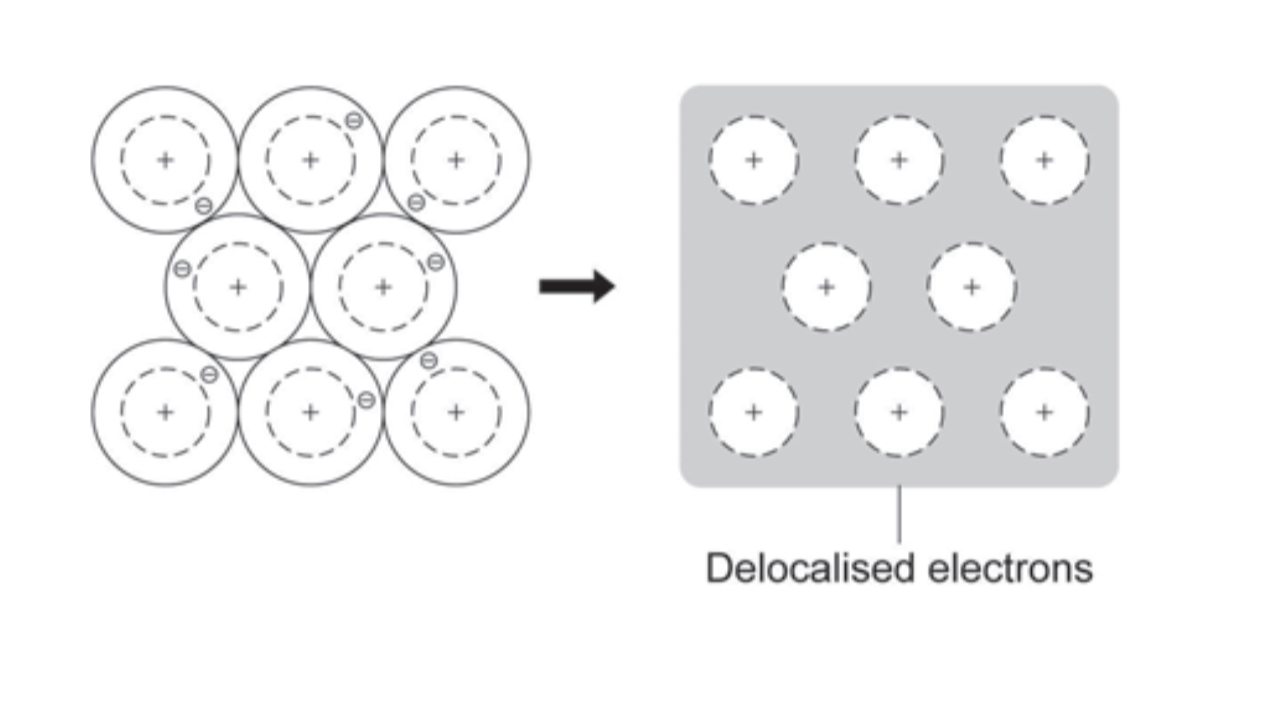
Structure of Metals
a giant lattice of positive metal ions
attracted to free (delocalised) electrons
Why can metals be Bent/shaped/stretched
into wires why are they soft?
metal atoms/ions are same size
layers are not distorted
layers slide
Why can metals conduct heat?
free (delocalised) electrons
move & transfer energy
through the structure
Why can metals conduct electricity?
Delocalised/free electrons
move & carry charge
through the structure
Why do metals have a high mpt/bpt?
giant lattice
lot of energy needed to break
strong metallic bonds
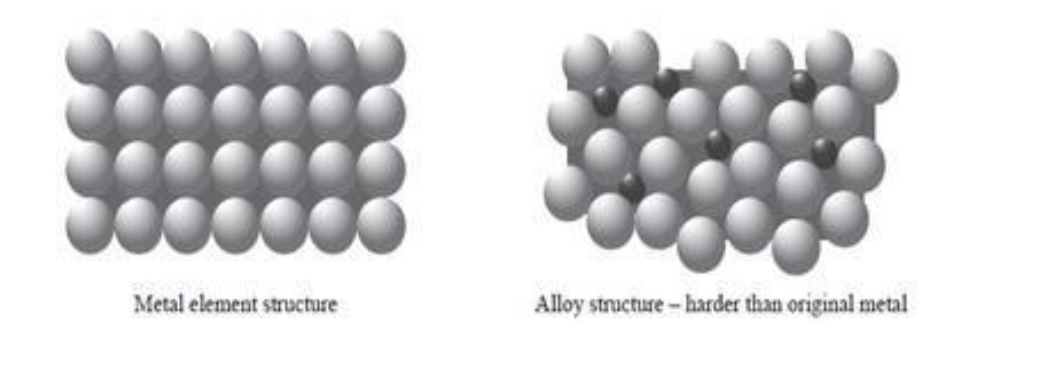
Pure metals are very soft/malleable (easily shaped) because:
atoms are the same size
layers not distorted
and can slide over each other
Pure metals have limited uses because…
-they aren’t strong (soft)
-Metals are made into alloys to make them harder and stronger
Alloys are…
mixtures of metals or a mixture of a metal & non-metal
eg carbon
Alloys are examples of a formulation because…
they are a mixture
designed as a useful product
Alloys are stronger than pure metals because:
other atoms are different size to original metal atoms
so layers of metal atoms are distorted
and cannot slide over each other
Why do molecules have low mpts and bpts?
simple molecules
little energy needed to break
weak intermolecular forces
Why do molecules not conduct
electricity?
no free electrons
to carry charge
through the structure
Diamond and Graphite are both
allotropes of carbon (different pure forms of the same element)
Diamond - description of structure
giant lattice of C atoms
each bonded to 4 others
by covalent bonds
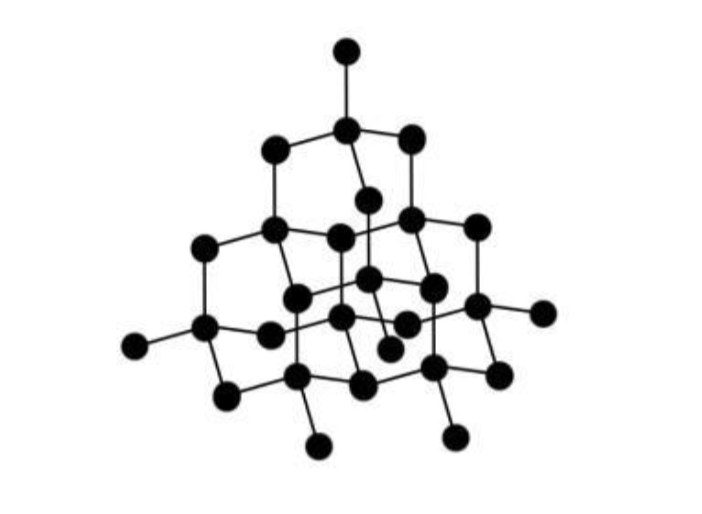
Why does diamond have a High mpt?
giant lattice
lot of energy needed to break
strong covalent bonds
Why is diamond hard?
each C atom bonded to 4 others
by strong covalent bonds
atoms cannot move
Why does diamond NOT conduct electricity?
no free (delocalised) electrons
to carry charge through the structure
Uses of diamond (related to properties)
Drill bits (very hard, high mpt)
Cutting other diamonds (very hard)
Graphite - Description of
structure
giant lattice of C atoms
each bonded to 3 others
by covalent bonds
weak forces between hexagon layers
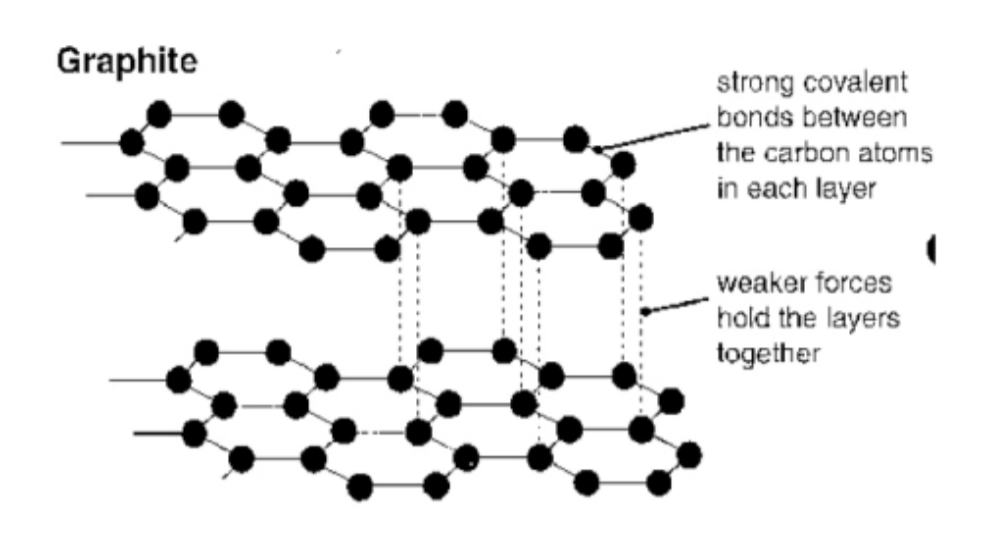
Why does graphite have a high mpt?
giant lattice
lot of energy needed to break
strong covalent bonds
Why is graphite soft?
weak forces between layers
so layers slide
Why does graphite conduct electricity?
Delocalised/free electrons
move & carry charge
through the structure
Graphite Uses (related
to properties)
Pencil “lead” (soft)
Lubricating machinery (slippery)
Electric motor contacts (conducts electricity)
What are fullerenes?
Hollow molecules of C atoms mainly arranged in hexagons (but
some include rings with 5 or 7 C atoms)
1st fullerene discovered:
Buckminsterfullerene (‘Buckyball’)
‘Buckyball’ contains…
60 C atoms covalently bonded into a
sphere of hexagons & pentagons
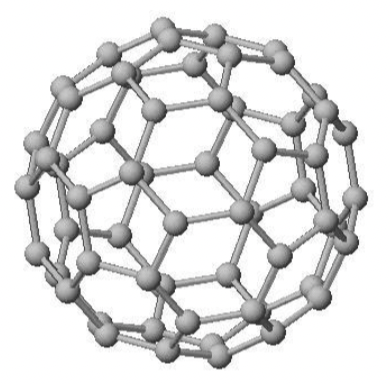
Why does Buckminsterfullerene have a low mpt/ bpt?
molecular structure/simple molecules
little energy needed to break
weak intermolecular forces
Why is Buckminsterfullerene slippery?
spherical shape
weak intermolecular forces
molecules can slide/roll
Why does Buckminsterfullerene conduct electricity?
Delocalised/free electrons
move & carry charge
through the structure
Carbon nanotubes are…
fullerenes in cylinder form
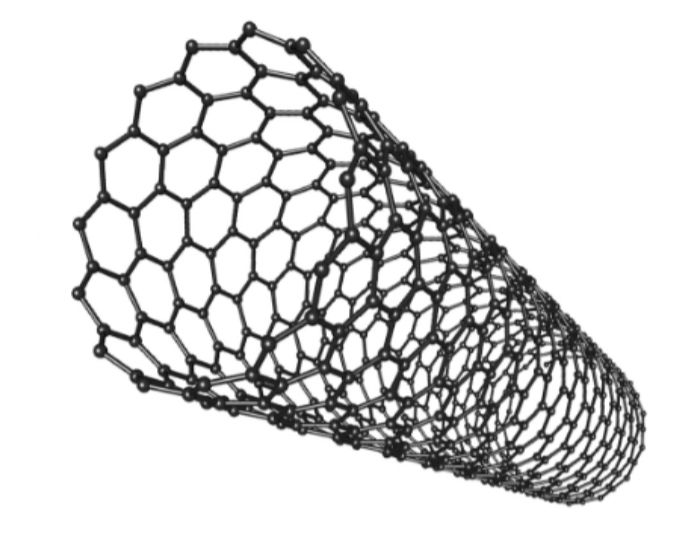
Why do Carbon Nanotubes have Relatively high mpt/bpt?
large molecules
lot of energy needed to break
strong covalent bonds
Why do carbon nanotubes resist stretching?
very long compared to their width
Uses of fullerenes
delivering drugs around the body (as they are hollow)
catalysts
strengthening other materials (as they do not break when
stretched)
electrical circuits
Surface Area to Volume Ratio
Surface Area / Volume
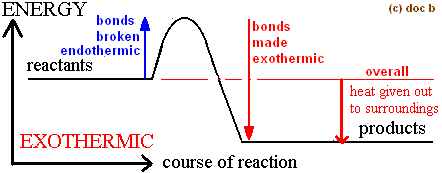
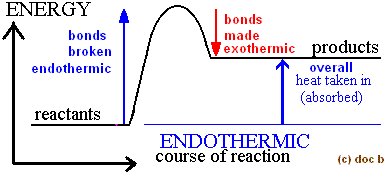
Energy Change
Bond breaking - Bond making
(Left - right)
Bond breaking (LHS) is..
endothermic (absorbs heat energy)
Bond making (RHS) is..
exothermic (gives out heat energy)
If overall energy change is negative
Exothermic
If overall energy change is positive
Endothermic
In terms of bonds, endothermic and exothermic reaction
Exothermic: Energy given out making new bonds > Energy needed (used) to make bonds
Endothermic: Energy given out making new bonds < Energy needed (used) to make bonds
Overall Exothermic
Overall Endothermic
Molecule size
molecules get larger
the weak intermolecular forces are stronger
more energy needed to break them
Examples of molecule size
group 7 mpt/bpt increase down the group
organic molecules getting longer
very long polymers compared to small molecules
What does this show 
atoms losing and gaining an electron to form ions with all electrons shown
What does this show
atoms losing and gaining an electron to form ions with only outer electrons shown.
Describing what happens in ionic bonding
-’who’ transferred electrons
-the charge it now has (periodic table!)
-’who’ gained electrons & how many
-what charge it now has (periodic table!)
-a reference to ‘oppositely charged ions strongly attract’
Describing what happens in ionic bonding EXAMPLE
Na transfers 1 outer electron to form Na^+
Cl gains 1 electron to form Cl^-
Na^+ & Cl^- strongly attract each other
 Describe the ionic bond
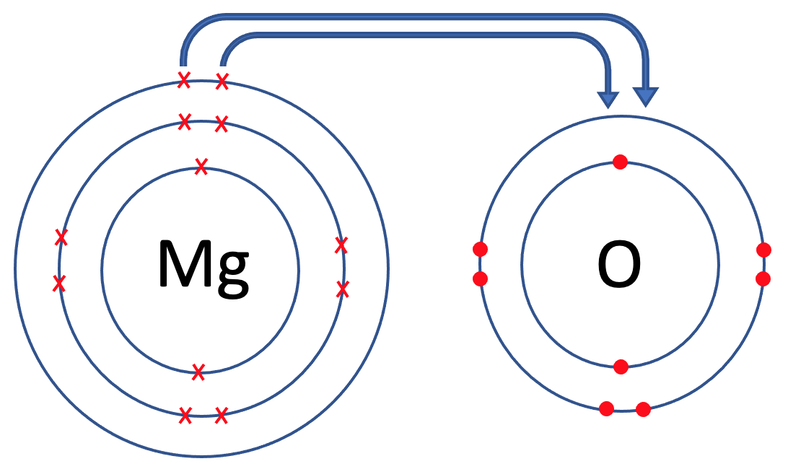
Describe the ionic bond
Mg transfers 2 outer electrons to form Mg²+
O gains 2 electrons to form O²-
Mg²+ & O²- ions strongly attract each other.
 Describe the ionic bond
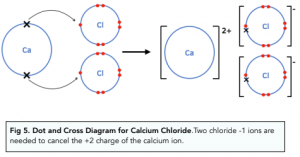
Describe the ionic bond
Ca transfers 2 outer electrons to form Ca²+
2 Cl each gain 1 electron to form Cl^-
Ca²+ & Cl^- ions strongly attract eachother
Ion charges: Group 1-3
-Group 1-3 elements (metals) transfer/lose electrons
-Their outer shells are now full like group 0 elements
-They are now positive ions because they have less electrons than protons
Ion charges: Group 6 & 7
-Group 6 & 7 elements (non-metals) accept/gain electrons
-Their outer shells are now full like group 0 elements
-They are now negative ions because they have more electrons than protons
For transition elements ion charge
Roman numeral in compound
Ions to know:
Cu²+
Fe²+
Fe³+
Ag^+
Zn²+
Copper (II)
Iron (II)
Iron (III)
Silver (I) or just silver
No Romans: +2
Molecular ions: Name charge and name of ion in compound:
NO3
OH
SO4
CO3
NH4
- nitrate
- hydroxide
-2 sulphate
-2 carbonate
+ ammonium
Ionic compound formula is correct
if + and - charges cancel
Elements in compounds exist as a
structure
In all ionic compounds..
ions are arranged in a giant lattice
oppositely charged ions are held by strong electrostatic forces of attraction
since structure is 3D, forces act in every direction
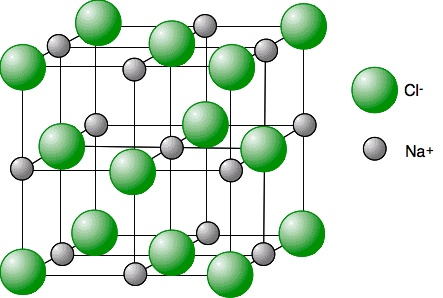 Physical properties of ionic compounds
Physical properties of ionic compounds
High melting point: Giant lattice, lots of energy needed to break strong forces of attraction between oppositely charged ions
Conducts electricity when melted or dissolved (solution): Ions not held in giant lattice so free to carry charge
Does not conduct electricity when solid: Ions held in giant lattice, not free to carry charge through the structure
How to recognise an ionic compound
-Melting points only give structure of a substance: high melting point = a giant lattice
-To tell if compounds are ionic/made of ions/have ionic bonding:
metal & non-metal in the names/formula eg NaF
conduct electricity only when melted or dissolved but not when solid
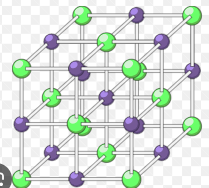
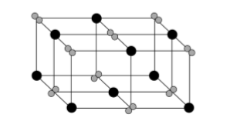
Limitations of ionic models
2-D model: only shows 1 later of ions/ doesn’t show where other ions are
3-D model: not to scale and large gaps between ions
Molecular ions need brackets if
there are 2 or 3 of that ion in a formula
Molecular Ions: Do the charges match + Formula of compound examples
Na+ & NO3-
Li+ & SO4-2
Mg²+ & NO3-
NH4+ & CO3-²
Yes - NaNO3
No. Need an extra Li - Li2SO4
No. We need an extra NO3 - Mg(NO3)2
No. We need extra NH4+ - (NH4)2CO3
To predict ionic formulae with a diagram…
Count & compare how many of one element compared to another
Grey = Iodine
Green = Lithium
Same number of Grey + (Li) and Green - (I)
For every 1 x Li there is 1 x I
So formula is just LiI
Predict ionic formulae
Grey = O
Black = Ti
Twice as many grey dots (O) as black dots (Ti)
1x Ti, 2 x O
TiO2
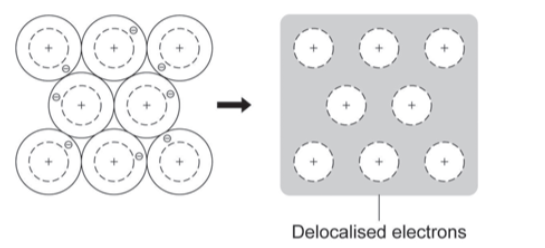
Structure of a metal
Metallic Bonding
the attraction between positive metal ions and free (delocalised) electrons
occurs in metallic elements and alloys
delocalised electrons free to move through structure, shared through structure so metallic bonds are strong
To describe the structure of any metal
a giant lattice of positive metal ions
attracted to free (delocalised) electrons
Properties of metals
Bent/shaped/stretched into wires/soft: metal atoms/ions are same size, layers are not distorted, layers slide
Conduct heat: delocalised electrons carry energy THROUGH THE STRUCTURE
Conduct electricity: delocalised electrons carry charge THROUGH THE STRUCTURE
High melting and boiling point: Giant lattice, lot of energy needed to break, strong metallic bonds
Limitations of covalent models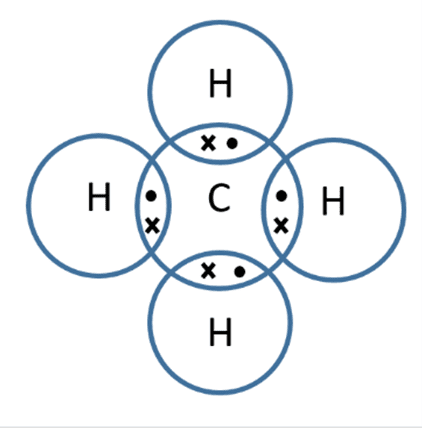
Dot and Cross - do not show shape of molecule/not 3 dimensional/ only 2 dimensional
Ball & stick - not to scale; doesn’t show electrons
Displayed formula - does not show shape of molecule/not 3d/ only 2d
Most molecules
are gases at room temperature (oxygen, nitrogen), some are liquid (water) & a few are solid (sulphur) but all have low/very low mpts and bpts
Physical properties of molecules
Low melting and boiling point - simple molecules, little energy needed to break, weak intermolecular forces
Do not conduct electricity - no free electrons, to carry charge, through the structure
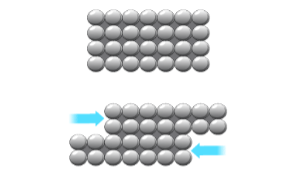
Pure metals are very soft/malleable
atoms are the same size
layers not distorted
can slide over eachother
Pure metals have limited uses because
they are not strong (soft) eg they cannot be used in construction
Metals are made into alloys to…
make them stronger and harder
Alloys
mixtures of metals or a mixture of a metal & non-metal eg carbon
Alloys are examples of
a formulation because they are a mixture designed as a useful product
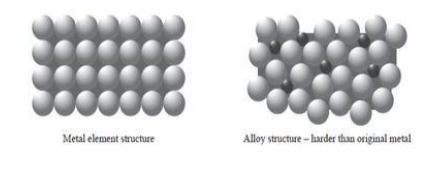
Alloys are stronger than pure metals because
other atoms are different size to original metal atoms
so layers of metal atoms are distorted
and cannot slide over each other.
Graphite - Description of structure
giant lattice of C atoms
each bonded to 3 others
by covalent bonds
weak forces between hexagon layers
Graphite - High Mpt
giant lattice
lot of energy needed to break
strong covalent bonds
Graphite - soft
weak forces between layers
so layers slide
Graphite - Conducts electricity
Delocalised/free electrons
move & carry charge
through the structure
Graphite - Uses (related to properties)
Pencil “lead” (soft)
Lubricating machinery (slippery)
Electric motor contacts (conducts electricity)
Graphene - High mpt/bpt (like
graphite)
giant lattice
lot of energy needed to break
strong covalent bonds