Social cells 3: G protein-coupled and enzyme linked receptors
1/17
Earn XP
Description and Tags
Ability of cells to receive and act on signals fundemental to life, all physiological signals are mediated through proteins
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
18 Terms
What is cell signalling?
Proteins recieving signals are called receptors
signals that are converted to a cellular response = signal transduction
cell signalling is specific: precise binding between the signal and the receptor - lock and key theory. it is also sensitive: receptors work at really low concentrations of 10-6 - 10-9 molar in the body (can be just as effective if we have only a few)
what is receptor activation?
receptors are either active state or inactive state dependent on their conformation (i.e. their shape)
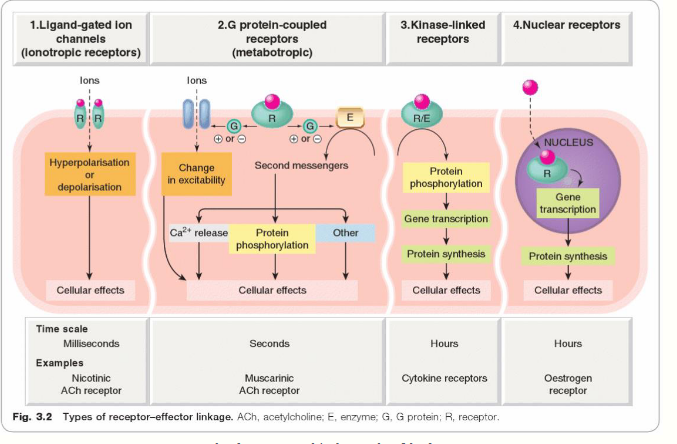
what are ligand-gated ion channels
work really quickly (in milliseconds)
involved in homeostasis, fast synaptic events, muscle contraction
ligand binds to receptor → conformation change allows transit of ions across membrane
no intermediate biochemical processes involved
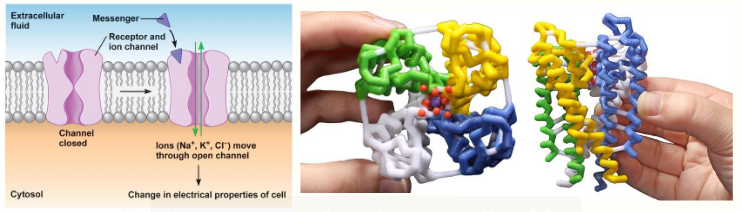
what is this showing
types and speed of recpeots
G protein coupled receptors
your nose works through G-protein coupled receptors - allows you to smell very quickly, work in seconds
over a 1/3 of medicines you prescribe act via GPCRs
more than 800 GPCRs in humans identified. the types involved in olfaction, regulation of immune responses, inflammation, homeostasis, autonomic nervous system, neurotransmission, growth and metastasis of tumours
receptor structure: 7 membrane-spanning a-helices sits within the bilipid membrane, crosses the membrane 7 times.
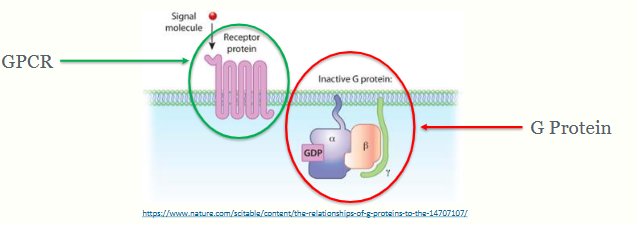
Closely associated with the recetor is the g protein - composed of 3 parts = trimeric G protein (not attached to receptor yet) → alpha, beta and gamma.
Attached to the g protein is a GDP molecule (guanine diphosphate), a way of transferring energy in the cell - at rest it is at a low energy state.
1) Signalling molecule/drug/ligand binds to receptor outside the cell.
2) Receptor changes shape and allows the G protein to connect to it to couple
3) GDP molecule gets replaced with GTP molecule = higher energy state.
4) G protein splits into 2 which is beta-gamma component and the alpha-GTP component interacts elsewhere (like to an enzyme) to trigger biological effect.
5) Inside the alpha unit is a phosphatase enzyme that takes away phosphate group to become alpha-GDP, that can then recombine with the G protein.
6) If the signalling molecule gets off the receptor, the receptor reverts back to its original shape, and the G protein dissociates from receptor again and system stops.
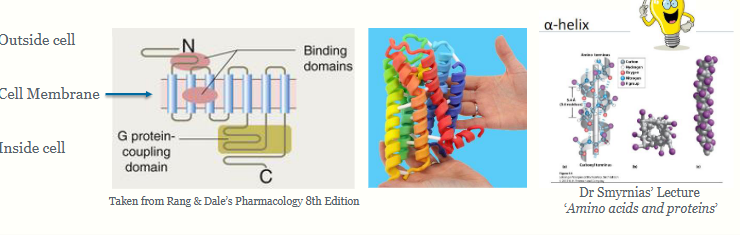
summarise G protein coupled receptors
apomorphine - drug you can inject to make the dog sick to vomit out the poison
Signal transducing molecules – i.e. they transfer extracellular signals into a cell, leading to a cellular response.
Inactive G proteins are trimers consisting of 3 subunits (α, β and γ) with a GDP molecule bound to the α subunit
G proteins are tethered to the cell membrane by the α and γ subunits, but are freely diffusible in the plane of the membrane
(what i basically wrote above)
1. Ligand binds to the GPCR at the ligand binding site and causes a conformational change in the receptor
2. This results in coupling of the receptor with a G Protein trimer; coupling
causes a conformational change in the G Protein
3. GDP on the α-subunit of the G protein dissociates and is replaced by intracellular GTP
4. The α subunit-GTP complex and the βγ-complex dissociate from the receptor and from each other. Both the α subunit-GTP complex and the βγ-complex are free to diffuse in the membrane and interact with enzymes or ion channels leading to signalling within the cell
5. GTP is hydrolysed to GDP by GTPase activity of the α-subunit
6. The resulting α subunit-GDP complex reunites with
the βγ-complex and signalling stops
what are the 4 main types of G protein?
usually changes on the alpha end
Gαs, Gαi, Gαq, Gα12/13
Diff a subunits show selectivity with respect to receptors and secondary messenger systems with which they interact
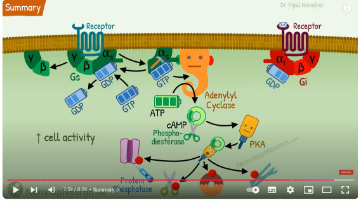
Gαs and Gαi produce, respectively, stimulation and inhibition
of adenylyl cyclase which produces cyclic AMP.
cAMP - secondary messenger that activates enzymes involved in energy metabolism, cell division and differentiation, ion transport, ion channels and contractile proteins in smooth muscle
part of the autonomic nervous system
examples of GPCR
stimulatory vs inhibitory
stimulatory: Gas (G protein alpha, stimulatory) → B1 adrenoceptor. stimulate adenylyl cyclase, increase cAMP activity, increase heart rate cos adrenaline bound to the B1 adrenoceptor
inhibitory: Gai (G protein alpha, inhibitory) → a2 adrenoceptor. inhibit adenylyl cyclase, reduce release of insulin by pancrease from adrenaline binding, so blood sugar stays high

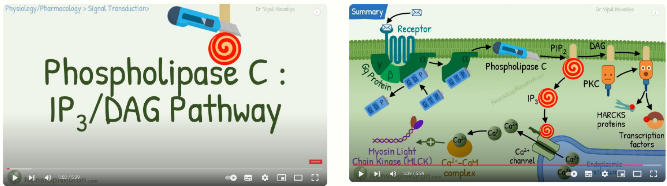
what is Gaq
it catalyses phospholipase C to produce inositol triphosphate (IP3) and diacylglycerol (DAG)
IP3 is a secondary messenger which leads to the release of intracellular Ca2+ - important role in the action of many hormones -> DAG activates protein kinase C, which plays an important role in many different aspects of cell function
e.g. GPCR linked to Gαq – Histamine (H1) receptor
it is an allergic disease, e.g. hay fever, stimulation of histamine receptors on mast cells leads to release of inflammatory mediators
what is Gα12/13
these subunits interact with guanine nucleotide exchange
factors (GEFs) for Rho family small GTPases.
involved in cytoskeletal dynamics, cell migration, proliferation, and survival.
Targeting this pathway is
a therapeutic interest for conditions like metastatic cancers and
inflammatory diseases.
• The primary effectors of Gβγ complexes are ion channels,
adenylyl cyclase, phospholipase C and PI3 kinase
what are enzyme-linked receptors?
receptors activated by mediators e.g. growth factors, cytokines and hormones
• Major role in growth & cell division, inflammation,
immune responses
• Main types are receptor tyrosine kinases (RTKs), receptor
serine/threonine kinases and cytokine receptors
• Time course of effect is minutes to hours
tell me more about kinase-linked receptors have a common structure
• Large extracellular binding domain connected via a single
membrane spanning α-helix to the intracellular domain
(tyrosine kinase enzyme)
• Activation of the receptor polypeptide chains usually involved
dimerization followed by autophosphorylation of tyrosine
residues. Relay proteins bind to the phosphorylated tyrosines
and activate downstream signalling
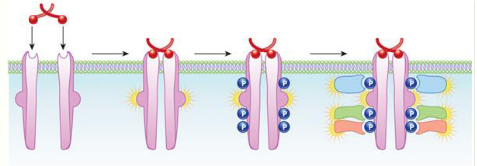
how are kinase-linked receptors activated or inactivated
1. Ligand binds to receptor and this leads to dimerization
2. The association between the 2 intracellular domains creates
and active kinase enzyme
3. Tyrosine residues are phosphorylated
4. The phosphorylated tyrosine residues act as docking sites for
other intracellular relay proteins (often enzymes) which are
then themselves activated
5. A cascade of events ultimately leads to a biological effect
6. The activity of the receptor is terminated by protein tyrosine
phosphatases
the central role of kinase cascades in signal transduction
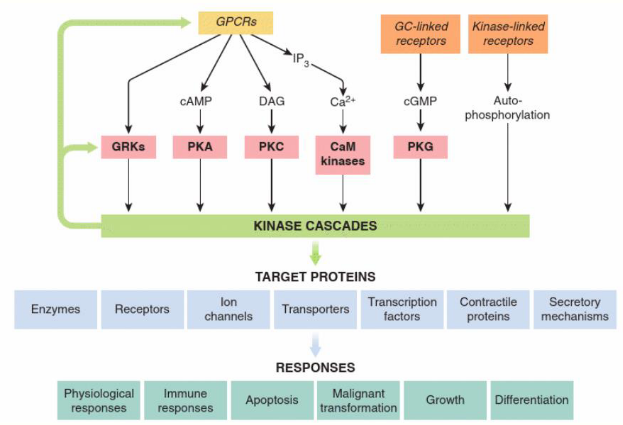
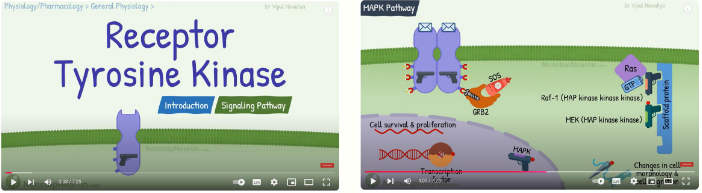
how do RTKs act through Ras and PI3-Kinase pathways
Ras and PI3-kinase pathways play a key role in cell division,
growth and differentiation
• Ras and PI3-kinase pathways important targets for anti-
cancer therapy
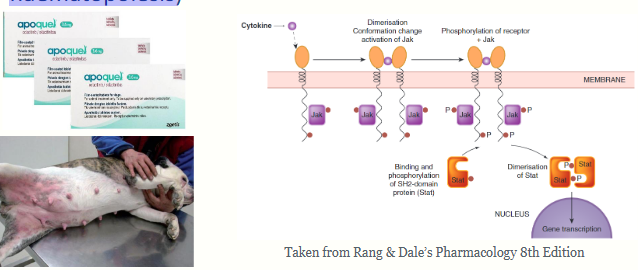
what is the Jak-Stat pathway?
The Jak-Stat pathway is activated by many cytokines and is
important in inflammation
• Oclacitinib is a Janus kinase (JAK) inhibitor - target cytokines
are those that are pro-inflammatory or have a role in allergic
responses/pruritus (also those involved in host defence or
haematopoiesis
what are the key points of GPCRs?
G Protein-coupled receptors are involved in a wide variety of
biological processes, including the action of hormones, energy
metabolism, cell division and differentiation, ion transport, ion
channels and contractile proteins in smooth muscle
• GPCRs act in a seconds timeframe
• All GPCRs have a common structure: 7 membrane-spanning
alpha helices.
• G proteins are comprised of three subunits (α, β, γ). There are
several types of G protein, which interact with different
receptors and control different effectors. Two such important
secondary messenger systems are the adenylyl cyclase and
phospholipase C systems
key points of enzyme-linked receptors
Enzyme-linked receptors are activated by a wide variety of
mediators including growth factors, cytokines and hormones
• Enzyme-linked receptors act in a minutes-hours timeframe
• Kinase-linked receptors have a common structure – a large
extracellular binding domain connected via a single membrane
spanning α-helix to the intracellular domain
• Ligand binding to the receptor leads to dimerization. The
association between the 2 intracellular domains creates and
active kinase enzyme. Tyrosine residues are phosphorylated.
The phosphorylated tyrosine residues act as docking sites for
other intracellular relay proteins (often enzymes) which are
then themselves activated. A cascade of events ultimately
leads to a biological effect. The activity of the receptor is
terminated by protein tyrosine phosphatase
• Receptor tyrosine kinases act through Ras and PI3-kinase
pathways. These pathways play a key role in cell division,
growth and differentiation and are therefore important targets
for anti-cancer therapy
• The Jak-Stat pathway is activated by many cytokines and is
important in inflammation