Grade 12 Biochemistry - Functional Groups
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
carbon chains
Carbon atoms that form a backbone.
Functional Groups
chemical groups attached to carbon skeletons that give compounds their functionality
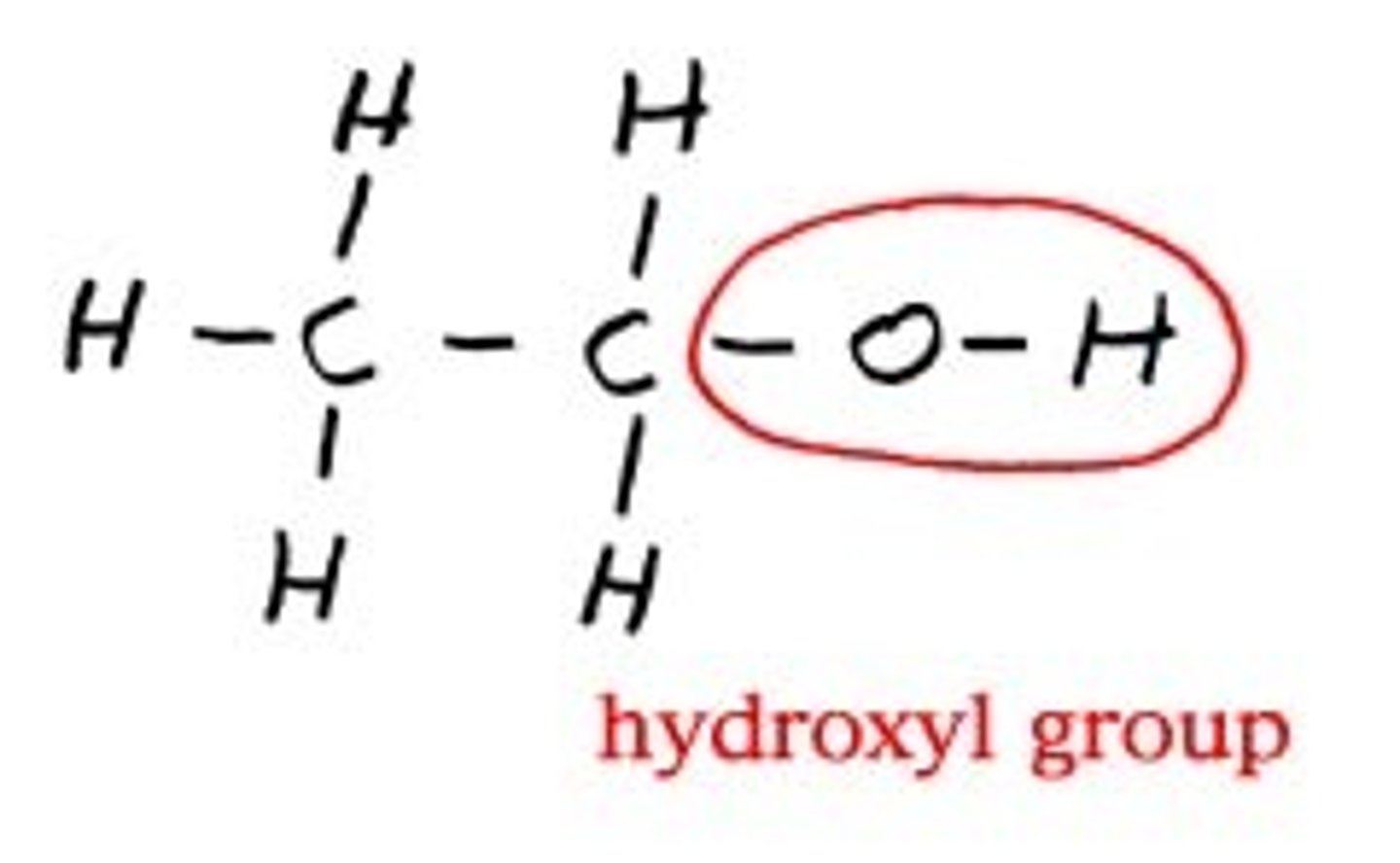
Hydroxyl Group Properties (-OH)
Polar, Hydrophilic
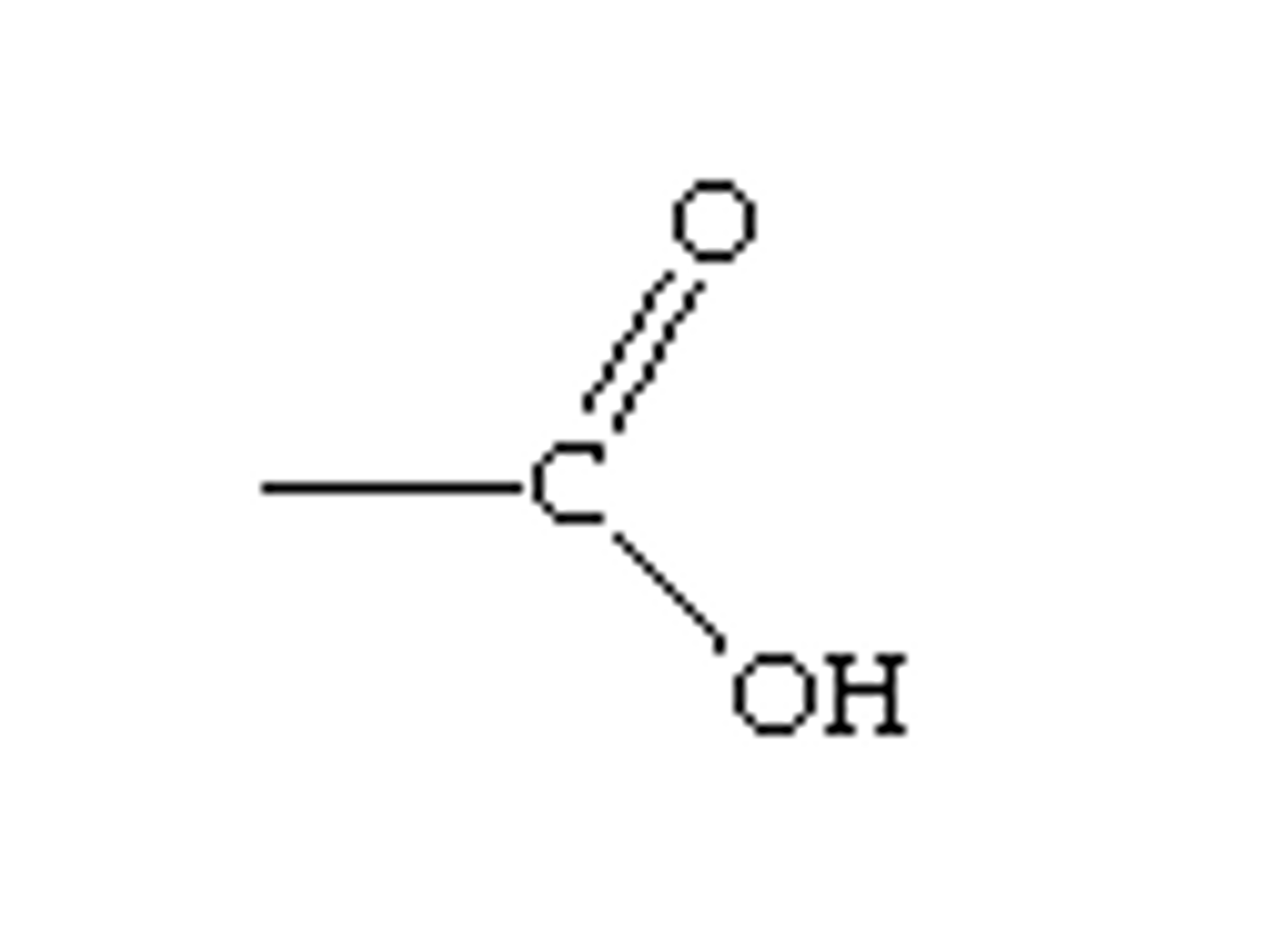
Carboxyl group Properties (-COOH)
release H+ therefore they are considered acidic.
Carbonyl group Properties (C=O)
Polar (Aldehyde and Ketone)
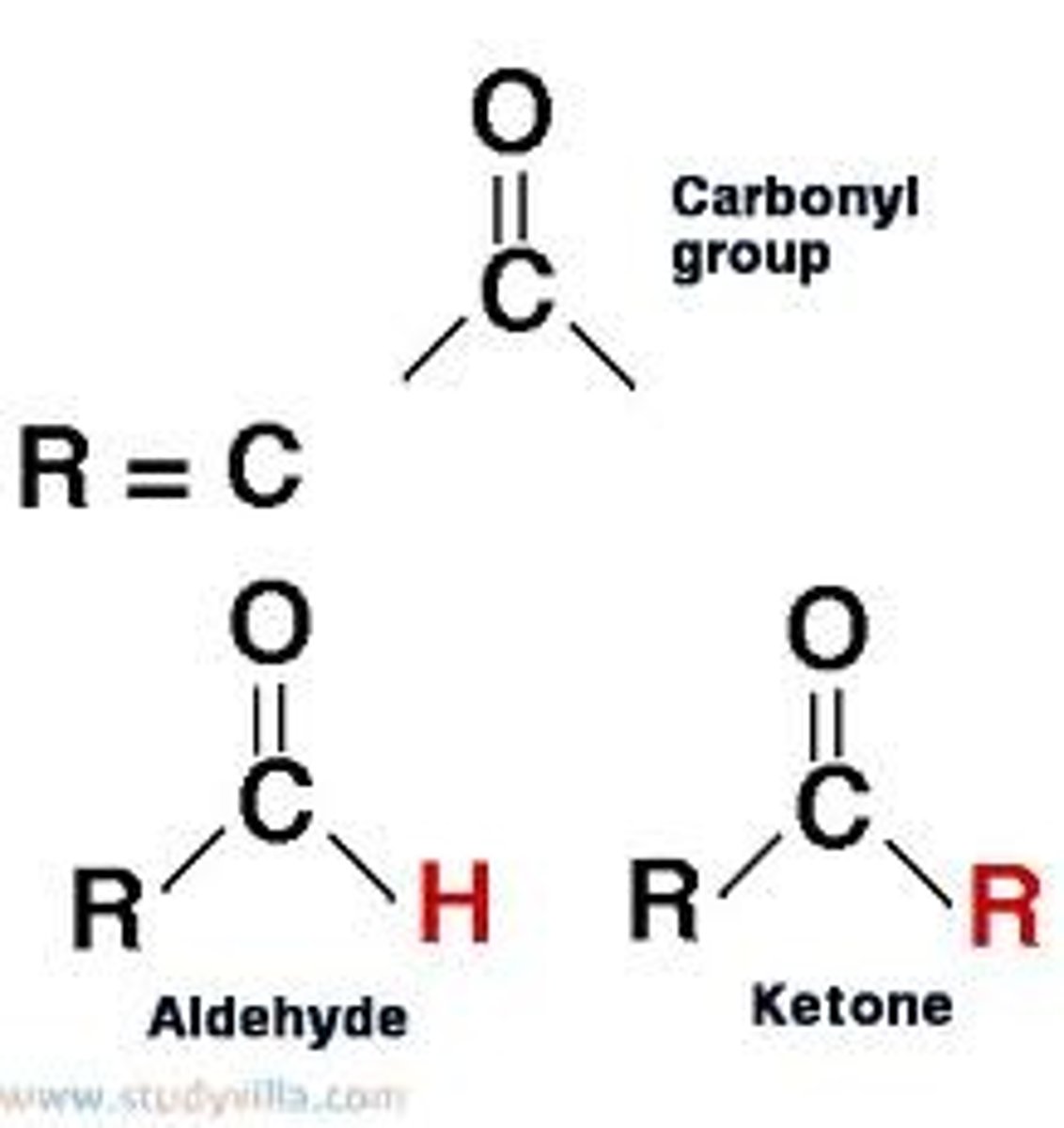
Aldehyde
An organic molecule with a carbonyl group located at the end of the carbon skeleton.
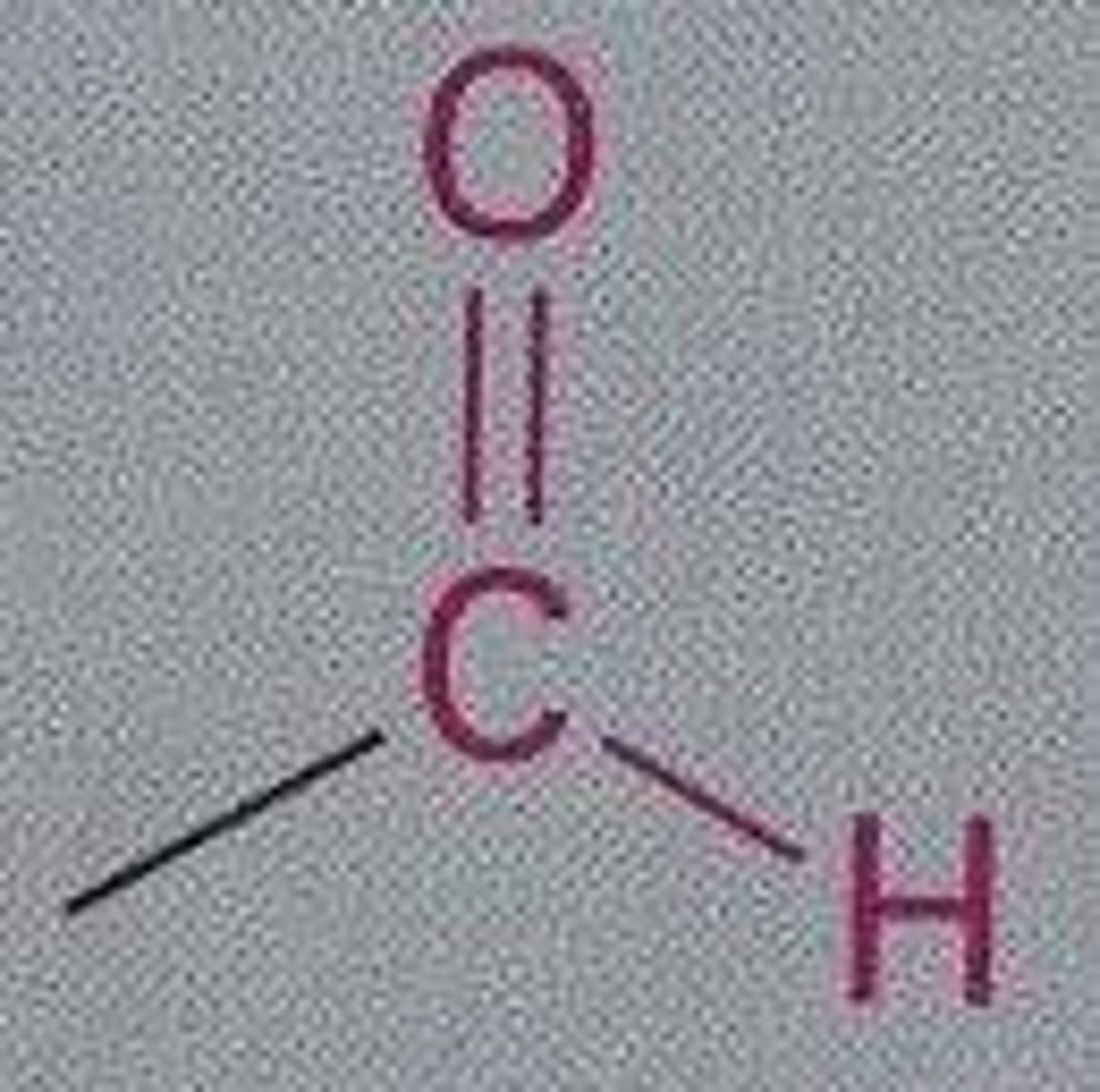
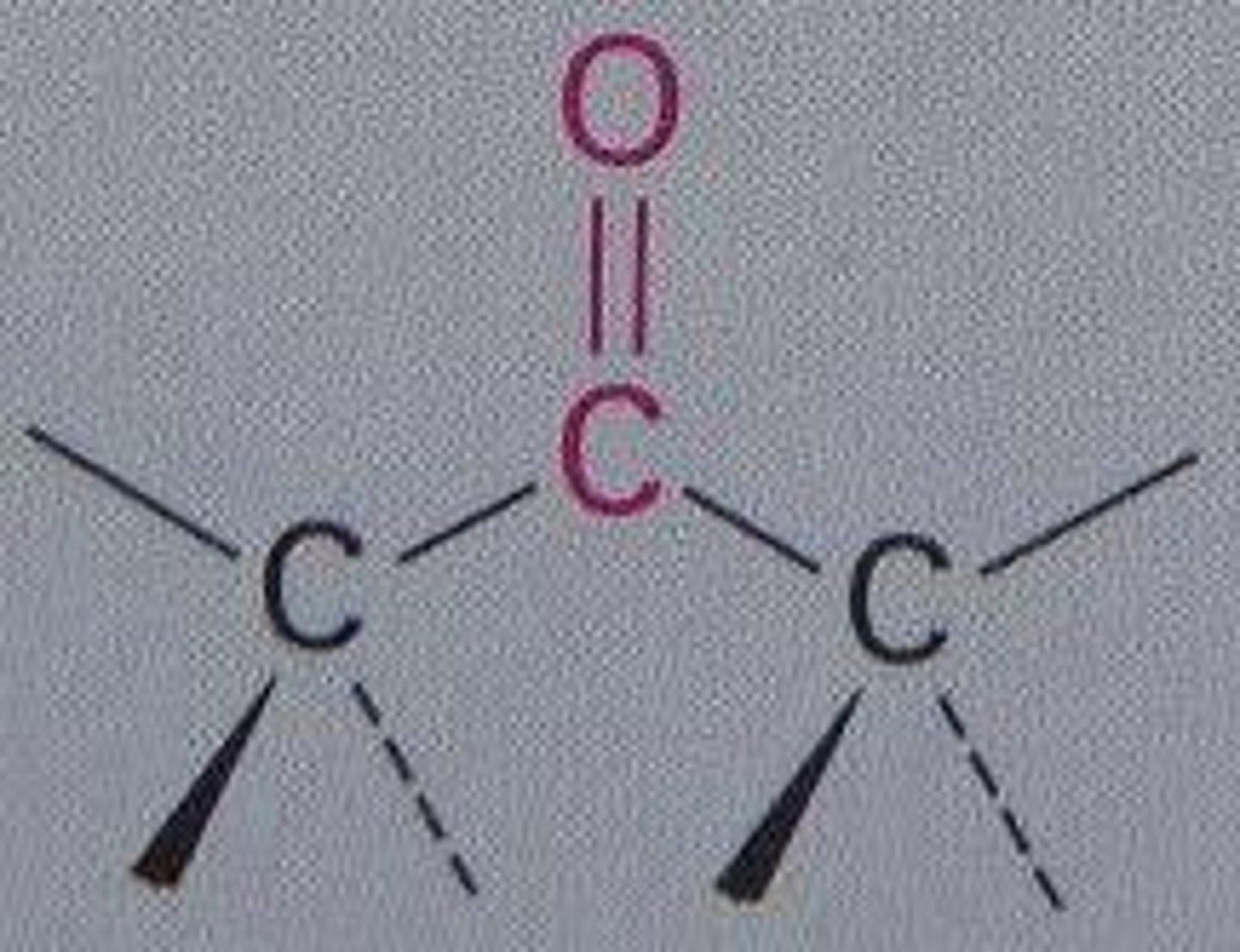
Ketone
An organic compound with a carbonyl group of which the carbon atom is bonded to two other carbons.
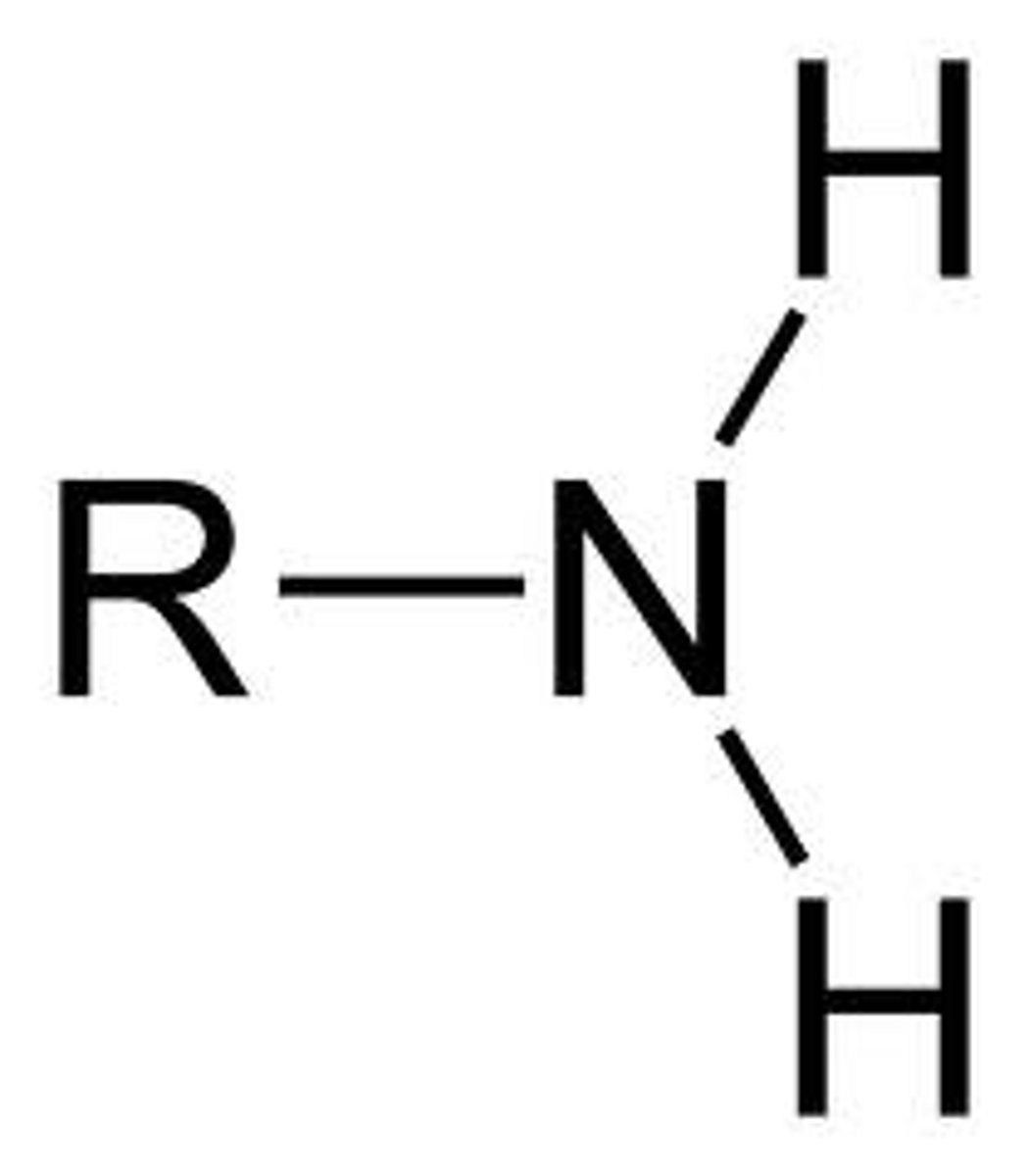
Amino group Properties (-NH2)
Has 1 N atom attached by covalent bonds to 2 H atoms. They can remove H+ so they are weak bases.
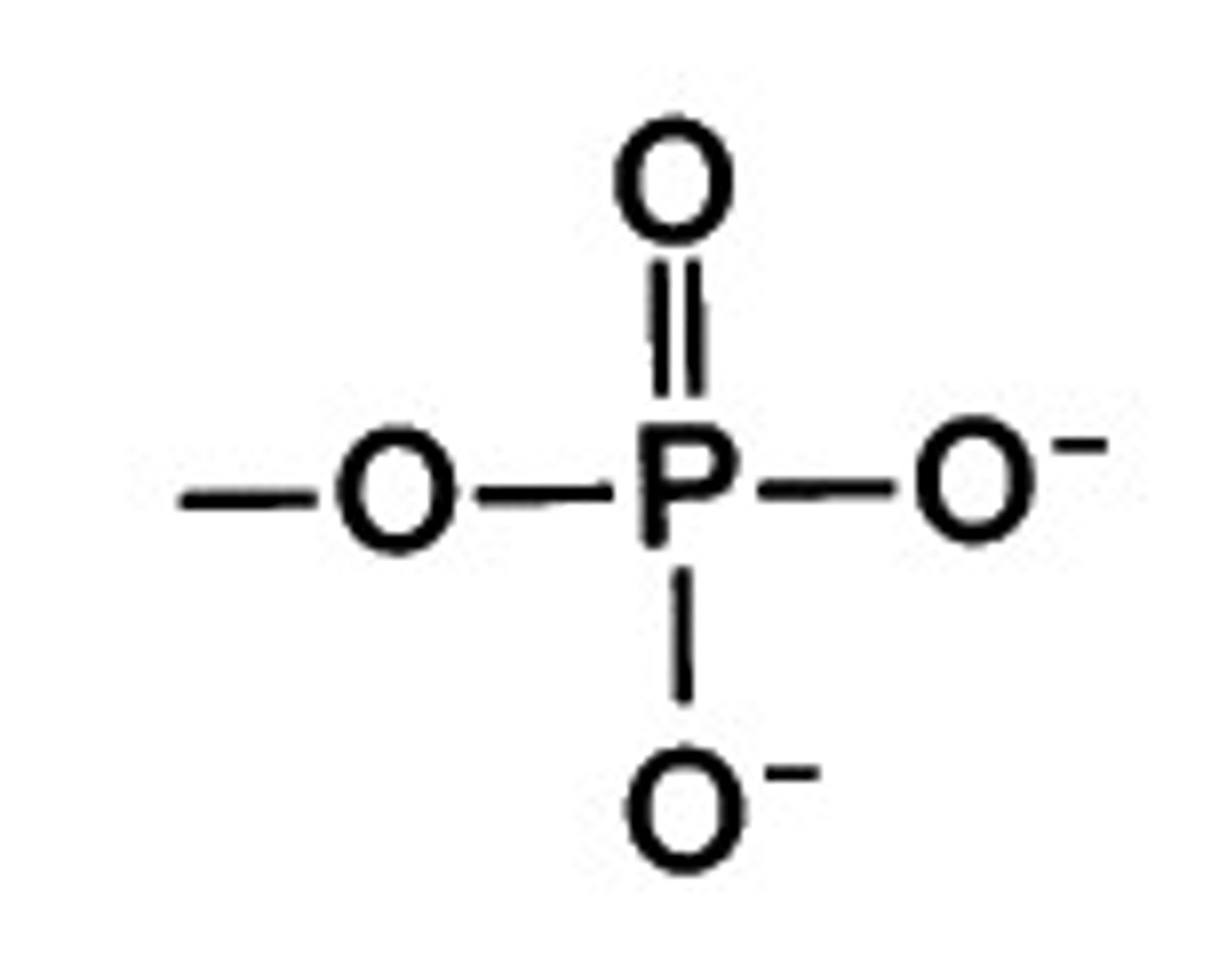
Phosphate Group Properties (-PO4)
Release H+ = acidic.
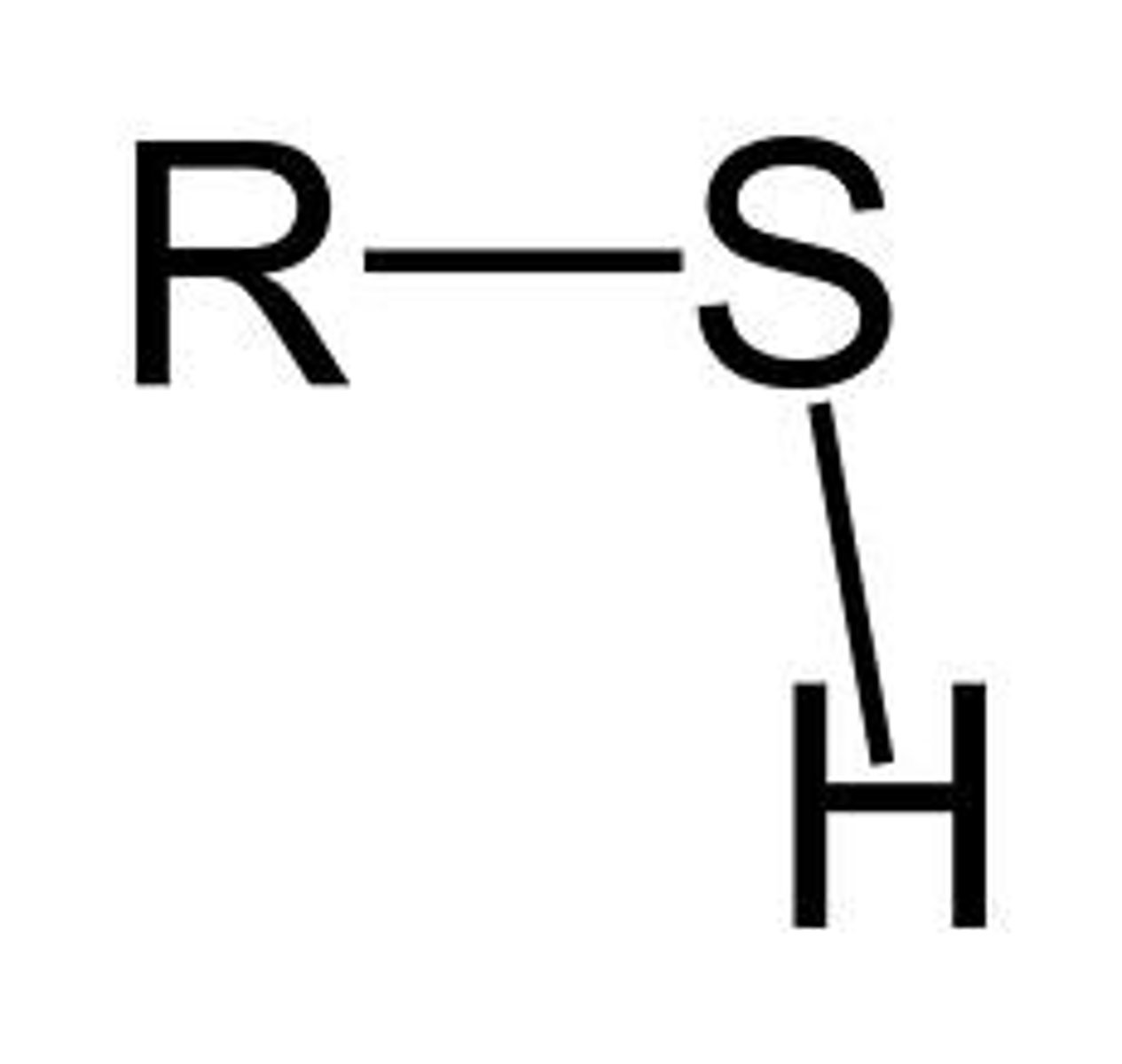
Sulfhydryl Group Properties (-SH)
Polar, consists of S atom, involved in protein structures
Methyl group Properties (-CH3)
Non polar covalent bond, has the ability to make any compound attached hydrophobic