Topic 12: Dilution of acids
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 6:44 PM on 5/18/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
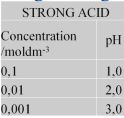
What is the trend for strong acids as they become more dilute?
They become less acidic with each dilution and instead become more alkaline, pH goes up by 1 each time.
2
New cards
Why do strong acids have a clear trend when it comes to dilution?
Non-reversible reaction when they dissociate into water
3
New cards
What is the trend for weak acids as they become more dilute?
Increases by a random amount of pH every time
Becomes more alkaline
4
New cards
Why do weak acids have no clear trend when it comes to dilution?
Reversible reaction when they dissociate into water -> equilibrium can shift constantly