Chapter 10
Interactions Between Particles
Phase change: A transition from one state of matter to another
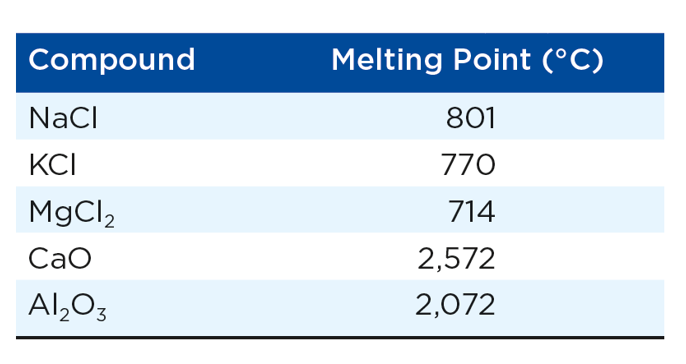
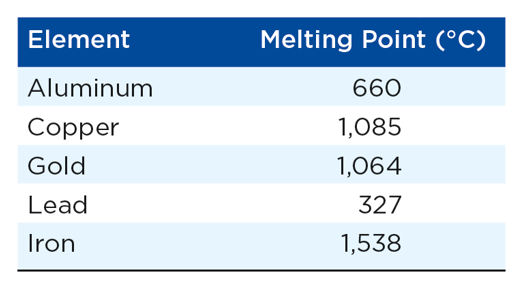
Stronger forces between particles - higher melting and boiling point
Ionic substances
Lattices: rigid frameworks of atoms, molecules or ions
Metallic Substances
Form lattices of tightly packed atoms
Electrons move easily between atoms
Shapes of metals are easily altered
- Malleable
- Ductile
Molecular Substances
Forces within molecules: Covalent bonds
Forces between molecules: Intermolecular forces
Covalent Networks and Polymers
Covalent networks: Lattices of covalent bonds that form giant molecules
Polymers: contain long chains of covalently - bonded atoms
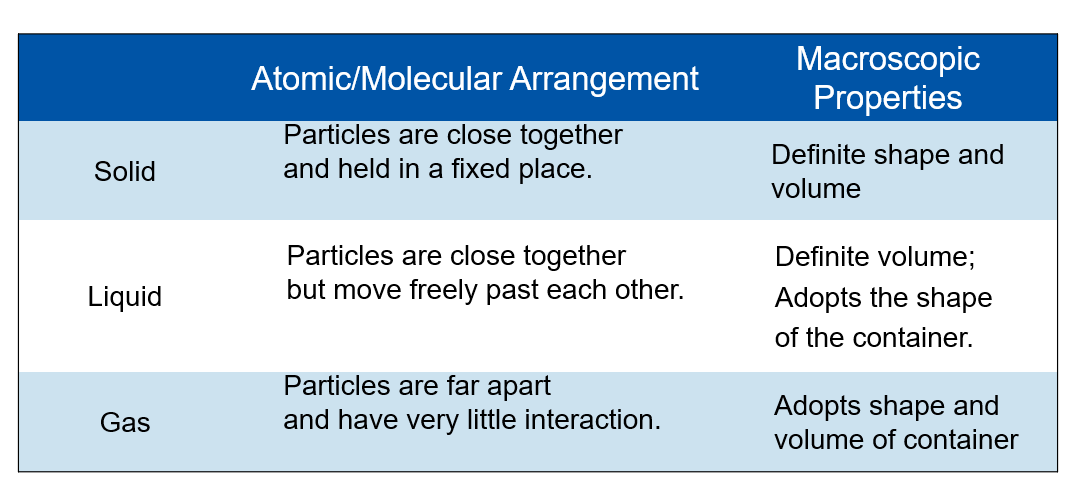
Describing Gases
Ideal Gas
Volume of particles is much less than container
Particles have no attraction for each other
- Connects Temp, Vol, Pressure
Pressure
The force that gases exert on their surroundings
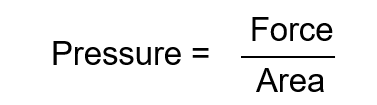
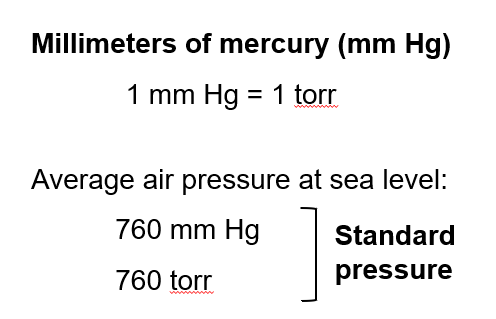
Measuring Pressure
Barometer: A device used to measure atmospheric pressure
Gauge Pressure: The difference between the compressed gas pressure and the atmospheric pressure

The Gas Laws
Boyle’s Law
The pressure and volume of a gas are ==inversely related==
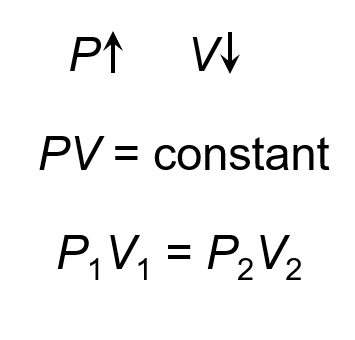
When working with Boyle’s law, we can use any units of pressure and any units for volume
Charles Law
At constant pressure, the volume of a gas is ==directly proportional== to its temperature
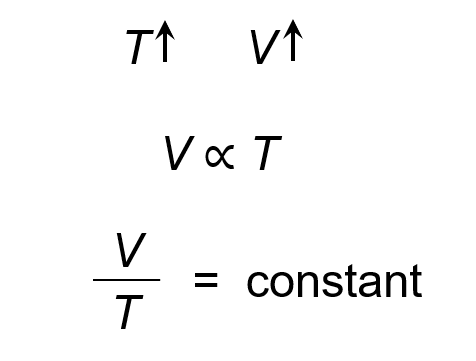
Absolute value = -273.15 + Celsius = Kelvin
Solving Problems with Charles Law
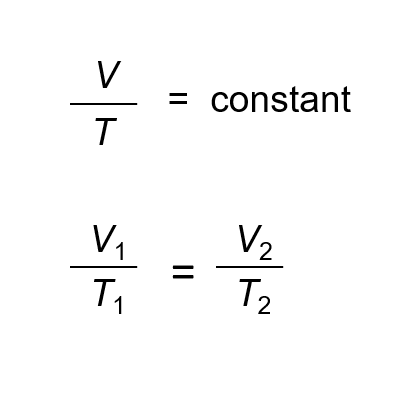
==Temperature = in KELVIN==
The Combined Gas Law
The temperature has to be in KELVIN
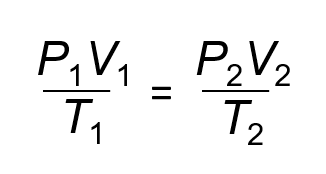
Avogadro’s Law
If temp and pressure are both constant, the vol of gas is proportional to the number of moles of gas present

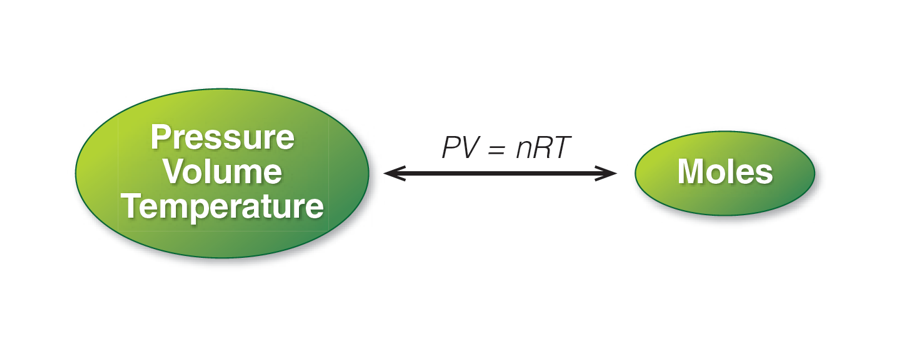
The Ideal Gas Law

Mixtures of Gases: Partial Pressures
The pressure caused by one gas in a mixture; Adding up all partial pressures gives the total pressure
Molecular View of the Gas Laws
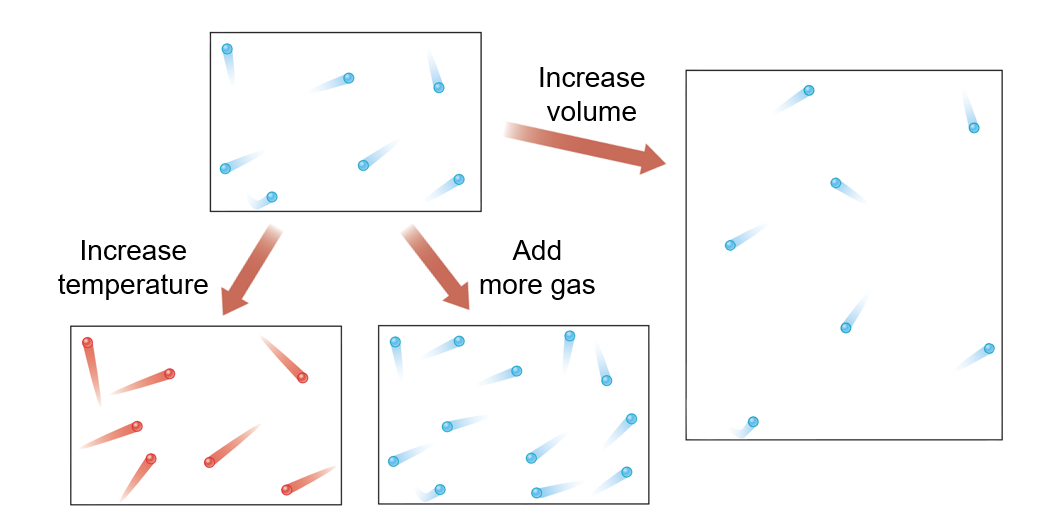
Diffusion
The spread of particles through random motion
Lighter particles diffuse more quickly
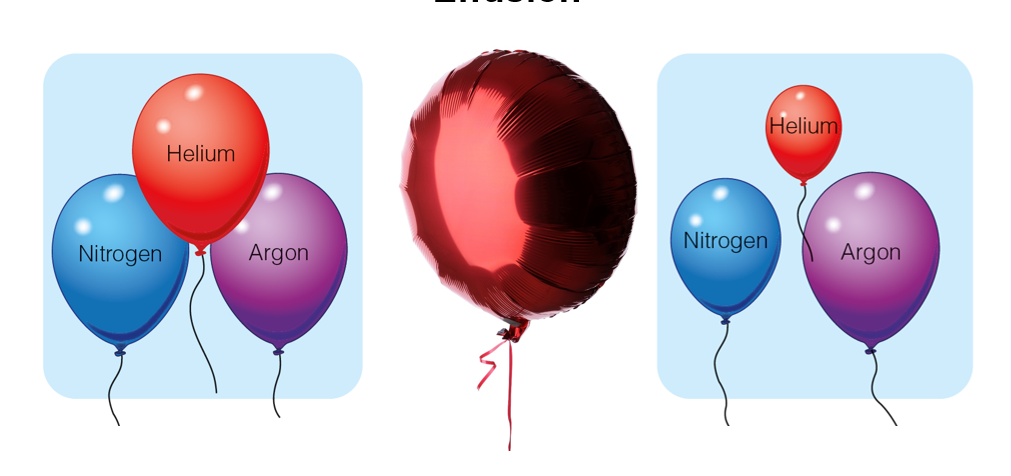
Effusion
The process of a gas escaping from a container
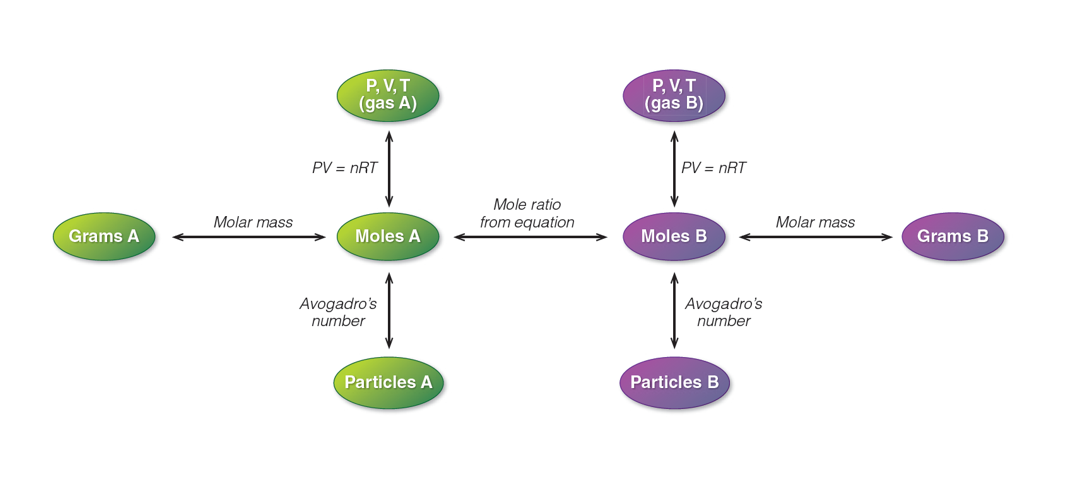
Gas Stoichiometry