Chem Mid term
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
140 Terms
The phlogiston theory of combustion is still considered correct today.
T or F
T
Theories can be tested and validated through experimentation.
T or F
T
Lavoisier developed the law of conservation of mass.
T or F
T
Which of the following items does NOT contain chemicals?
All of the above contain chemicals.
organically grown vegetables
insecticides
drain cleaner
air
All of the above contain chemicals.
Who discovered the atomic theory?
Antoine Lavoisier
none of the above
John Dalton
John Dalton and Antoine Lavoisier
Nivaldo Tro
John Dalton
A good definition of chemistry is:
none of the above
the science that seeks to understand what matter does by studying what atoms and molecules do.
the science that seeks to understand the interactions of molecules for the sake of advancing human control over nature.
the science that seeks to understand what living organisms do by studying the molecules that make up the organism.
the science that seeks to understand what the universe does by studying interactions of molecules with atoms.
the science that seeks to understand what matter does by studying what atoms and molecules do.
If you know the density of a liquid and its volume, the mass of the liquid may be calculated using the equation: m = V/d.
T or F
T
If you are given the mass and density of an object, you can calculate the volume by using the equation:
V = m/d.
T or F
T
The base unit of mass in the SI system is the kg.
T or F
True
The number 7.20 × 103 contains three significant figures.
T or F
T
The correct decimal representation of 1.201 × 10-7 is
0.0000001201
The correct prefix for the multiplier 1,000 is:
mega.
milli.
none of the above
micro.
nano.
none of the above
The correct number of significant figures in the number 0.027090 is
5
Metals expand to a larger volume when heated. If a piece of metal was heated, which one of the following statements would be TRUE?
none of the above
The newly calculated density value of the metal would not change from the initial value.
The newly calculated density value would decrease.
The newly calculated density value would increase.
The mass of the metal would also increase.
The newly calculated density value would decrease.
An object weighing 1.840 kg has a volume of 0.0015 m3. What is the density of the object in g/cm3?
1.2
The common English unit in which the speed of an automobile is expressed is miles/hr. What is the set of base SI units for speed?
m/s
Which of the following would NOT be considered a correct conversion factor?
12 eggs = 1 dozen eggs
5 cents = 1 nickel
100 pennies = 1 dollar
1 dozen eggs = 12 eggs
1 pair of shoes = 1 shoe
1 pair of shoes = 1 shoe
A lead ball has a mass of 55.0 grams and a density of 11.4 g/cm3. What is the volume of the ball?
4.82 mL
Determine the answer to the following equation with correct number of significant figures:
13.96 - 4.9102 + 71.5 = ________
80.5
How many cm3 are there in 1.25 ft3?
3.54 × 104
Our English springer spaniels like our neighbors’ children and could play with them all day. We thought our dogs will never dislike any children they will ever meet. Last month when we took our dogs on a trip, we met some German tourists in a park. The dogs loved playing with the foreign family’s children. Our English springer spaniels like all children.
"Our English springer spaniels like all children" is our _____
conclusion
Our English springer spaniels like our neighbors’ children and could play with them all day. We thought our dogs will never dislike any children they will ever meet. Last month when we took our dogs on a trip, we met some German tourists in a park. The dogs loved playing with the foreign family’s children. Our English springer spaniels like all children.
Allowing the dogs to play with the German family's children is the____
experiment
Our English springer spaniels like our neighbors’ children and could play with them all day. We thought our dogs will never dislike any children they will ever meet. Last month when we took our dogs on a trip, we met some German tourists in a park. The dogs loved playing with the foreign family’s children. Our English springer spaniels like all children.
"Our dogs will never dislike any children they meet" is the
hypothesis
Our English springer spaniels like our neighbors’ children and could play with them all day. We thought our dogs will never dislike any children they will ever meet. Last month when we took our dogs on a trip, we met some German tourists in a park. The dogs loved playing with the foreign family’s children. Our English springer spaniels like all children.
"Our English springer spaniels like our neighbors' children" is a(n)
observation
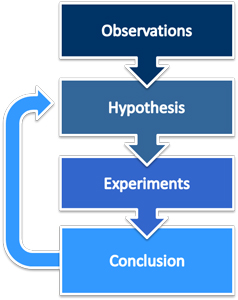
Order of scientific method
The star closest to our Solar System is moving away from Earth at a high speed.
law
theory
observation
observation
A body in motion stays in motion unless acted upon by a force.
theory
law
observation
law
The universe began as a cosmic explosion called the Big Bang.
law
theory
observation
theory
A stone dropped from an altitude of 450 mm falls to the ground in 9.6 s
law
theory
observation
observation
What is wrong with the statement, "It is just a theory"?
A scientific theory is the best current explanation of a phenomenon that has been validated by years worth of experiments.A scientific theory is the current explanation of any phenomenon that was given by two or more world well-known scientists.
This statement is true; a theory is something that was suggested without any evidance.
A scientific theory is the best current explanation of a phenomenon that has been validated by years worth of experiments.
proven statement explaining the objective facts.
observation,
law
hypothesis
theory
law
an approval, suggesting evidence.
observation,
law
hypothesis
theory
hypothesis
- a system of ideas or principles which are a set of generalized provisions.
observation,
law
hypothesis
theory
theory
- an organized perception of phenomena with the aim of studying them.
law
observation
hypothesis
theory
observation
The first step in acquiring scientific knowledge is often ______
making an observation
performing an experiment
refining a theory
developing a hypothesis
making an observation
Which of the following is characteristic of scientific theories?
Scientific theories cannot be modified.
Scientific theories are based upon informed opinions.
Scientific theories are easily discarded.
Scientific theories are validated by experimental evidence
Scientific theories are validated by experimental evidence
According to the scientific method, what is a law?
A tentative interpretation or explanation of the observations
A short statement that summarizes a large number of observations.
A fact that can never be refuted.
An initial guess with explanatory power.
A short statement that summarizes a large number of observations
Which term best represents the models that explain and give the underlying causes for conclusions drawn from many years of experimental evidence?
observation
theory
hypothesis
law
theory
A general statement that summarizes a number of similar observations is a(an)
experiment
theory.
scientific law
hypothesis.
scientific law
What is the difference between a law and a theory?
A scientific_____is a brief statement that summarizes previous observations and can be used to predict the results of future related experiments.
A scientific____explains why the observations occur.
law; theory
Enter the number 1340000 in scientific notation.
1.34x106
Enter the number 0.000354 in scientific notation.
3.54 × 10-4
Type the number 3.14×103 in standard notation.
3140
Type the number 8.75×10-4in standard notation.
000875
23.56 how many sig figs
4
how many sig figs 500.01
5
23.560 how many sig figs
5
0.0078 how many sig figs
2
Determine the number of significant figures in the measurement 6.07
3
Determine the number of significant figures in the measurement 0.000450
3
Round the value 23.731 gto three significant figures.
23.7 g
6×0.222×7.0
round the answer appropriately.
9
orig ans 9.324
0.411+9.9+0.8
round the answer appropriately.
11.1
orig ans 11.111
In addition or subtraction, the result has the same number of decimal places as the quantity with the fewest decimal places.

16.83/15 − 0.46
and round the answer appropriately.
Express your answer numerically using the appropriate number of significant figures or decimal places.
16.83 / 15= 1.122
1.122-0.46= 0.662
round
0.7
How many grams are in 337 mg?
g to mg = divided by 1000
0.337g
How many milliliters are in 9.00 dL?
dL to mL = x 100
Conversion factor for g to mL
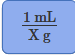
Conversion factor for mL to g
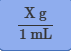
Conversion factor for L to kg
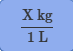
Conversion factor for kg to L
Aluminum has a density of 2.70 g/mL Calculate the mass (in grams) of a piece of aluminum having a volume of 331 mL.
894 g
A piece of aluminum with a volume of 331 mLmL will have a mass of 894g . This can be calculated by multiplying 331 mL by the conversion factor of 2.70 g/mL
M= D x V
deci factor
10-1
deka factor
101
hector factor
102
kilo
103
mega
106
centi
10-2
milli
10-3
micro
10-4
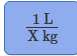
Iron has a density of 7.86 g/cm3(1 cm3=1 mL). Calculate the volume (in dL) of a piece of iron having a mass of 3.97 kg. Note that the density is provided in different units of volume and mass than the desired units of volume (dL) and the given units of mass (kg). You will need to express the density in kg/dL (1 cm3 = 1 mL) before calculating the volume for the piece of iron.
5.05 dL
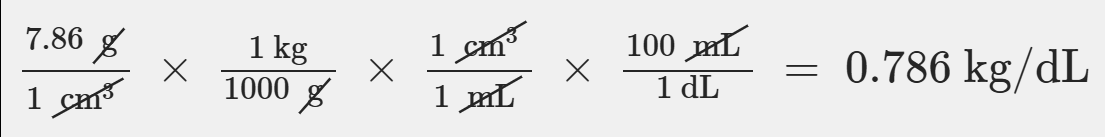
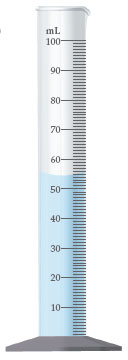
54.3 mL
50 mL
54.36 mL
54 mL
54.3 mL
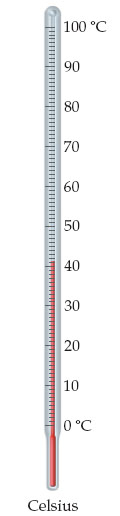
41.3 ∘C
41 ∘C
40 ∘C
41.34 ∘C
41.3 ∘C
mg
Convert 2.51×10−6g to each unit.
2.51×10−3 mg
cg
Convert 2.51×10−6g to each unit.
2.51×10−4 cg
ng
Convert 2.51×10−6g to each unit.
2.51 × 103

A student gains 1.6 lb in two weeks. How many grams did he gain?
7.3×102g

2.3ft2 to in2
330in2

2.7yd2 to ft2
24ft2
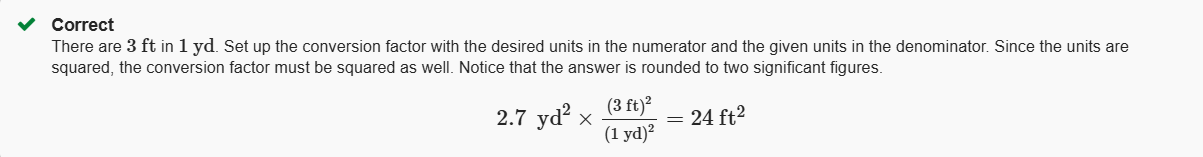
2.8 m2 to yd2
3.4yd2

A sample of an unknown metal has a mass of 35.4 g and a volume of 3.11 cm3
Identify the metal.
ρ= 11.4g/ cm3
d=m/v
Classify each of the following as an observation, a law, or a theory.
When a metal is burned in a closed container, the mass of the container and its contents do not change.
observation
law
theory
observation
Classify each of the following as an observation, a law, or a theory.
Matter is made of atoms.
observation
law
theory
theory
Classify each of the following as an observation, a law, or a theory.
Matter is conserved in chemical reactions.
observation
law
theory
law
What is the difference between a hypothesis and a theory?
Before a hypothesis is well established, it is referred to as a scientific theory.
A scientific theory and a hypothesis are the same.
Before a scientific theory is well established, it is referred to as a hypothesis.
Before a scientific theory is well established, it is referred to as a hypothesis.
In 1999, NASA lost a $94 million orbiter because one group of engineers used metric units in their calculations while another group used English units. Consequently, the orbiter descended too far into the Martian atmosphere and burned up. Suppose that the orbiter was to have established orbit at 155km and that one group of engineers specified this distance as 1.55×105m. Suppose further that a second group of engineers programmed the orbiter to go to 1.55×105ft
How low did the probe go?
The orbiter would have attempted to establish an orbit 47.2km above Mars.
The orbiter would have attempted to establish an orbit 108km above Mars.
The orbiter would have attempted to establish an orbit 108ft above Mars.
The orbiter would have attempted to establish an orbit 47.2ft above Mars.
108km
The orbiter would have attempted to establish an orbit 47.2km above Mars.
(77.96 - 9.74) × 45.6 = 311
3.11 × 103
(8.1 ×105 ÷ 2.948 ×102) + 121 =
2.9 × 103
(5.4 ×103 - 1.53 ×103) ÷ 34.5
1.1×102
Perform the following calculation to the correct number of significant figures:
When the measured numbers 3.270 and 0.081 are multiplied, the numerical answer should be expressed as
0.26
Perform the following calculation to the correct number of significant figures:
1.15×0.522×1.344×0.006÷3.5
1 × 10-3
Perform the following calculation to the correct number of significant figures:
4.297×3.99835÷89.9
0.191
Perform this multiplication to the correct number of significant figures: 63.8×0.0031×13.11
2.6
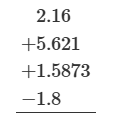
7.6

131.89
When 0.27 is subtracted from 5.6000, the answer should be rounded to:
5.33
Two is the least number of decimal places in the numbers used.
2.9 km2 to m2
2.9 × 106 m2

1.7 cm3 to m3
1.7×10−6 m3
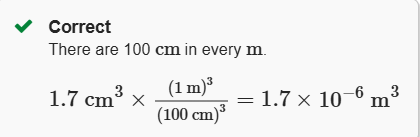
2.5 mm3 to m3
2.5 × 10-9
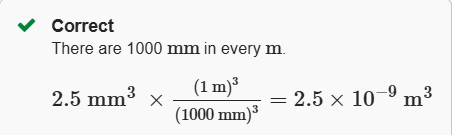
order for electron dot diagram
clockwise manner starting on the right. The next electron is drawn underneath the symbol, and then on the left side. Finally, an electron is drawn above the symbol.
Although this is generally the order in which electrons are drawn, it is not the absolute method.
What is the Lewis structure of the covalent compound that contains one carbon atom, one oxygen atom, and two hydrogen atoms?
formaldehyde (CH₂O)