equilibrium in industrial processes
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
6 Terms
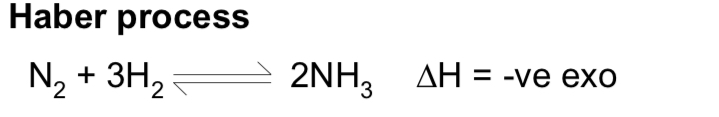
what is the haber process? what ate the conditions & compromises?
T = 450C
P= 200-100ATM
catalyst= iron
low temp gives good yield but slow rates so temp used is compromises
high pressure gives good yield and high rate, too high a pressure leads to too high energy costs for pumps to rpoduce the pressure
what is the catalyst used in the contact process? what are the compromises?
V2O5 CATALYST
low temp gives good yield but slow rates so the compromise is that a moderate temp is used
high pressure gives slightly better yield and higher rate nut this would lead to high energy costs to produce the pressure
why are catalysts good in industry?
they speed up the rate, allowing lower temp to be used (lower energy costs) but they have no effect on equilibrium
why are unreacted reactants recycled?
recycling unreacted reactants back into the reactor can improve the overall yields of all these processes
give 3 reasons why product is removed before reaching the maximum conc equilibrium
the equilibrium position will shift to the night
OR
this will favour forward reaction
(1)(in an equilibrium) removal of product decreases rate of back reaction / rate of formation of reactants)
(1)time to attain / reach equilibrium may be too long
(1)
• unreacted reactants can be recycled
Why are catalysts useful in industry?
Catalysts increase the rate of reaction moving toward equilibrium by providing an alternate reaction pathway with a lower activation energy
Allows milder conditions to be used so there are lover energy costs