Organic Chemistry- Sn1, Sn2, E1, E2
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
3 > 2 >>>> 1….. Methyls cannot react
Sn1, E1, E2
Methyl > 1 > 2 > …….3 cannot react
Sn2
Polar protic solvents: polar solvents with protons to donate
Sn1, E1
Polar aprotic solvents: polar solvents that do not have an H bond donor
Sn2, E2
High temp
Sn1, E1, E2
low temp
Sn2
Form carbocations +
Sn1, E1
Concerted (happens at once)
Sn2, E2
Stepwise
Sn1, E1
Concerted (happens all at once)
Sn2, E2
Racemic mixtures
Sn1
Lots of possible reactions, double bond formation with methyl or hydride shift, and always give the most stable product
E1
Stereoinversion (2 products)
Sn2
I- > Br- > Cl- > F-
Sn1
F- > Cl- > Br- > I-
Sn2
NEt3
Polar aprotic solvent
DMSO
Polar aprotic solvent
acetone
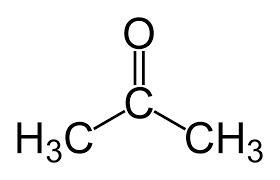
Polar aprotic solvent
acetonitrile
Polar aprotic solvent
DMF
Polar aprotic solvent
H20
Polar protic solvent
MeOH
Polar protic solvent
EtOH
Polar protic solvent
acetic acid
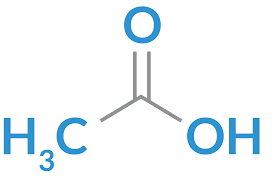
Polar protic solvent
NaH, NaNH2
NEt3, PO tert-butyl, lithium diisopropylamide
Strong base weak Nu
NaOH, NaOMe, NaOEt
Strong base strong Nu
R-NH2, R-S, HS, CN, N3, I, Br, Cl, RSH, H2S
weak base strong Nu
H20, MeOH, EtOH, acetic acid
weak base weak Nu
H3PO4, H2SO4
strong acid terrible Nu
Strong base Weak Nu
1: E2
2: E2
3: E2
Strong base Strong Nu
1: E2 (30%) / Sn2 (70%)
2: E2 (70%) / Sn2 (30%)
3: E2
Weak Base Strong Nu
1: Sn2
2: Sn2 (90%) / Sn1 (10%)
3: Sn1
Weak base Weak Nu
1: no rx
2: Sn1 (60%) / E1 (40%)
3: Sn1 (50%) / E1 (50%)
Strong acid Terrible Nu
1: E1
2: E1
3: E1
-H20 is LG (dehydration)