17-18. B cell activation and antibody production
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
what is clonal selection of B cells?
selection and activation of specific B cells based on antigen recognition
what types of antigens can B cells bind?
native protein antigen
polysaccharides
lipids
nucleic acids
chemicals
(much broader recognition than T cells)
what were nude (athymic) mice used to study?
defective thymus → lack T cells
used to determine role of T cells
what are thymus-dependent antigens? what type of antigen is thymus-dependent?
antigens dependent upon helper T cells to induce antibody production
without T cells, B cells are not activated
proteins
what are thymus-independent antigens? what types of antigens are thymus-independent?
antigens that do NOT need helper T cells to induce antibody production
polysaccharides, lipids (non-protein antigens)
what do follicular B cells respond to? what do they produce?
recognize T-dependent antigens
protein antigen + helper T cell
produce isotype-switched, high-affinity antibodies and long-lived plasma cells
broad antigen receptor diversity
where are follicular B cells found?
spleen, other lymphoid organs (ex. lymph nodes)
what types of B cells recognize T-independent antigens (polysaccharides, lipids)?
marginal zone B cells → important for bloodborne antigens
B-1 B cells
limited antigen receptor diversity
where are marginal zone B cells found?
spleen (major) and other lymphoid organs
where are B-1 B cells found?
mucosal tissues, peritoneal cavity
what do T-independent B cells produce?
mainly IgM and short-lived plasma cells
not producing long-lived plasma cells or immunological memory
not switching Ab isotype
how does a secondary antibody response differ from a primary antibody response?
2° is a stronger, more accelerated (quicker) response
presence of memory cells → higher starting levels of antibody
switch Ab production from IgM to IgG (higher affinity for antigen)
where are memory B cells found? where are long-lived plasma cells found?
usually found in secondary lymphoid tissues
most long-lived plasma cells are found in bone marrow
what is required for B cell activation?
2 signal-model of activation → simultaneous engagement of:
B cell receptor complex
co-receptor complex (or TLR)
need inflammation/danger signal with innate immune activation to activate naive B cells
what are the components of the B cell receptor complex?
B cell receptor (IgM) → interacts with antigens
Igα & Igβ → associated proteins that provide signaling
what are the components of the co-receptor complex?
CR2
CD19
CD81
how does complement activation contribute to B cell activation?
C3b (produced by complement) bound to a microbe binds to CR2 (part of co-receptor complex)
what can provide the second signal if complement is not activated?
TLR recognizing PAMPs
what are the results of B cell activation?
biochemical signal transduction
endocytosis/internalization of antigen → antigen-specific APC
what effects does B cell receptor-mediated signal transduction have on the B cells?
increased survival, proliferation
interaction with helper T cells (present antigens via MHC II)
increased responsiveness to cytokines
migration from follicle to T cell zone (increased expression of CCR7)
antibody secretion
what is CCR7?
receptor that is receptive to chemokines in T cell zone — CCL19/21
expression increased in activated B cells
expression downregulated in activated T cells
linked recognition
T-dependent antigen must contain both B and T cell epitopes
epitopes must be on the same antigen, though they are not necessarily the same epitope
very important for vaccine development
how do B cells present antigens to helper T cells?
B cell recognition of native protein antigen
receptor-mediated endocytosis of antigen
antigen processing and presentation (via MHC II pathway)
(CD4+) T cell recognition of antigen
how do dendritic cells contribute to T-B cell interaction?
DCs activate naive helper T cells → activated T cell downregulates CCR7 → starts to migrate out of T cell zone, toward B cell zone at the same time B cell is moving toward T zone
helper T-cell dependent activation of B lymphocytes
effector T cells produce cytokines that further amplify B cell response and direct isotype switching
TCR-MHC II signaling → T cell produces CD40 ligand & B cell expresses CD40 molecule
CD40 signaling is critical → modifies B cell transcription
what are consequences of CD40 or CD40L deficiency?
no secondary response
hyper-IgM syndrome (cannot switch isotype)
immunocompromised
no macrophages activation (also needs CD40 signaling)
poor antibody response
what happens to B cells after interacting with T cells?
some differentiate into short-lived plasma cells → produce Abs quickly to control infection
some head back to follicles and start germinal center reaction
how do extrafollicular helper T cells become follicular helper cells (Tfh)?
after interacting with B cells, some helper T cells start expressing CXCR5, which is attracted to CXCL13 produced by follicular dendritic cells in the B cell zone → go to follicles with B cells and form germinal centers
what 3 processes are involved in the germinal center reaction?
affinity maturation
somatic hypermutation of Ig genes
isotype switching (also occurs outside of germinal centers)
generation of memory B cells & long lived plasma cells
what process(es) occur in the dark zone of the germinal center?
high levels of B cell proliferation
what process(es) occur in the light zone of the germinal center?
somatic hypermutation
affinity maturation
isotype switching
affinity maturation
process that leads to increased affinity of antibodies for a particular antigen
result of somatic hypermutation of Ig genes, followed by selective survival of B cells producing antibodies with the highest affinity for the antigen
how is activation-induced deaminase (AID) induced? what process(es) is it involved in?
induced by CD40 signals
key role in somatic hypermutation (and isotype switching)
during somatic hypermutation, in what part of the antibody do the mutations accumulate?
in or near the CDRs (variable region)
how can we experimentally show that the mutations increase affinity?
measure Kd (dissociation constant) → how well Ag & Ab stick together (how easy it is to separate once bound)
↓ Kd = ↑ affinity
measure Kd of antibodies during primary and subsequent immune responses → decreased Kd as mutations accumulate → increasing affinity
what is the function of activation-induced deaminase (AID)? what is the significance of this reaction for somatic hypermutation?
converts dC (deoxycytidine) → dU (deoxyuridine)
dU is an unnatural nucleotide in DNA, so it is removed and replaced with dT (deoxythymidine)
C → T change in DNA sequence is an error prone mechanism, leading to even more mutations
what two survival signals do B cells need in order to not undergo apoptosis after somatic hypermutation?
antigen recognition
CD40 signaling from helper T cells (CD40/CD40L)
where is antigen found in the germinal center?
localized on follicular dendritic cells (FDCs)
Fc receptors → Ag-Ab complexes
C3b/C3d (complement) receptors → C’-Ag-Ab complexes
is antigen freely available to all B cells in the germinal center?
no → limited amount of antigen in germinal center
competition for antigen becomes more intense as immune response goes on → selecting higher and higher affinities
compete with other antibodies (including Ab on FDCs) to obtain antigens from FDCs
what component(s) of the antibody contribute(s) to isotype switching?
heavy chain only! (light chain does not contribute to isotype)
how is isotype switching induced/directed?
cytokines produced by CD4+ helper T cells (or mucosal tissues)
IgG switching is induced by what cytokine?
IFN-γ (produced by TH1)
IgE switching is induced by what cytokine?
IL-4 (produced by TH2)
IgA switching is induced by what cytokine? where is this cytokine produced?
TGF-β (transforming growth factor beta) (& others not emphasized)
produced in the mucosal tissues (MALT), which are constantly exposed to antigens → need to suppress immune response
what is a switch region?
region of DNA located at the 5’ end of each constant region gene segment
become transcriptionally active depending on cytokines present
what is the mechanism of switch recombination?
switch region at a particular heavy chain locus becomes transcriptionally active (cytokines) → targeted by AID
AID converts dC → dU, which is then replaced with dT; creates ds DNA breaks
intervening DNA is deleted; new constant region is next to VDJ region
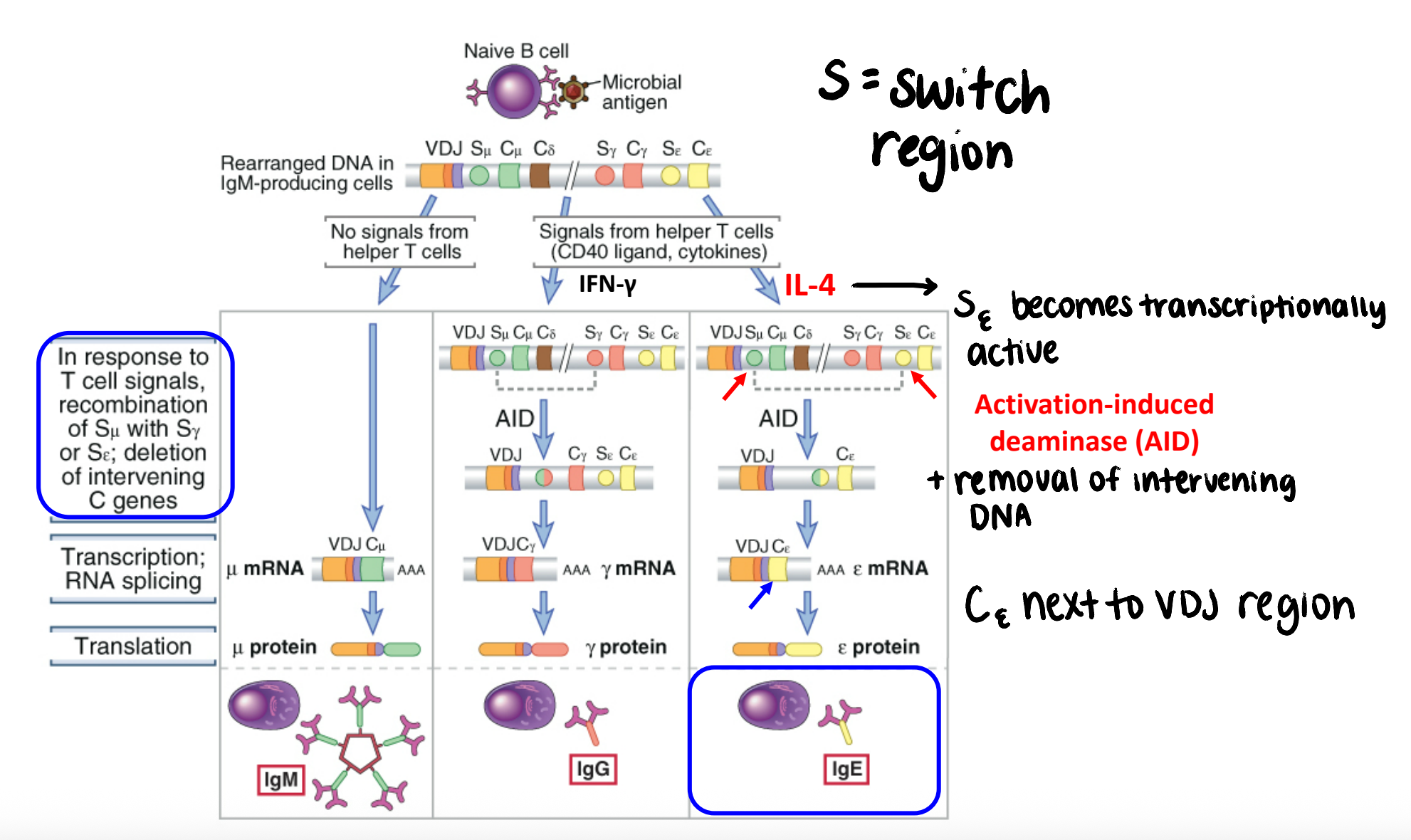
does switch recombination affect antigen specificity?
NO → VDJ region unchanged during this process
switch recombination specializes the effector functions (Fc region)
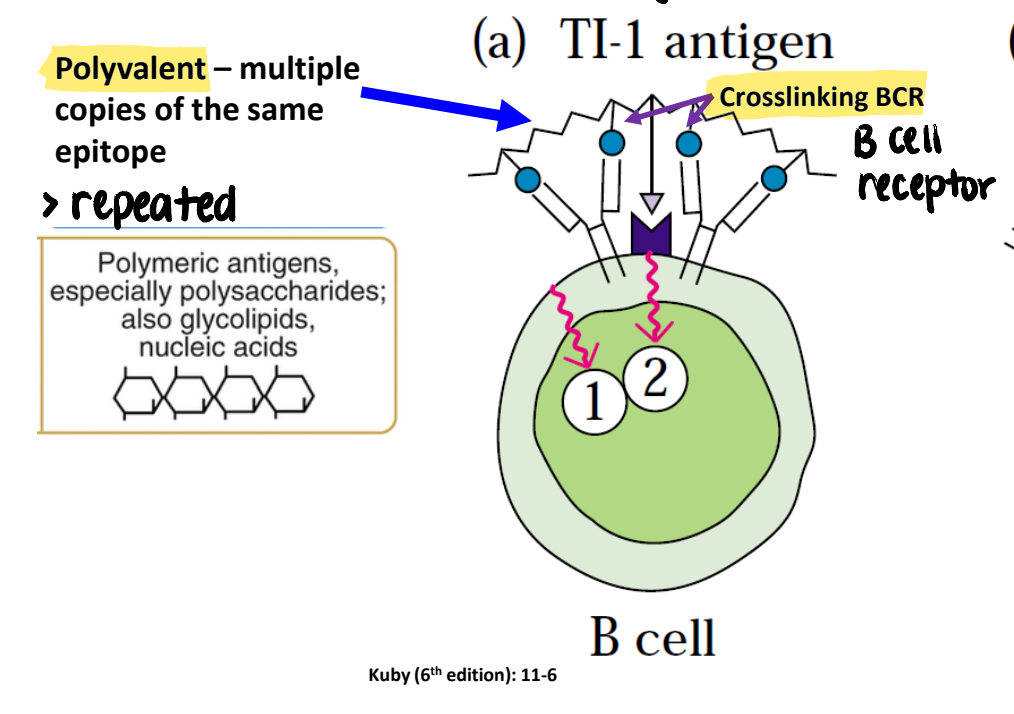
what is a polyvalent antigen? what types of antigens tend to be polyvalent?
multiple copies of the same epitope on the same antigen
thymus-independent antigens
how do polyvalent antigens interact with B cells?
induce maximal crosslinking of membrane Ig on B cells, without a need for T cell help
how does the overall antibody response differ for T-independent antigens (compared to T-dependent antigens)?
low level switching to IgG
little to no affinity maturation
produces short-lived plasma cells
no memory B cells produced
**no immunological memory produced
how can we induce a very strong antibody response to polysaccharides?
conjugate a t-independent antigen to a t-dependent antigen → stimulate isotype-switched, high affinity antibody responses to the t-independent antigen
B cells can then present processed antigen to CD4+ T cells via MHCII to do T cell dependent processes
(create a T-dependent response from a T-independent antigen)