The pH scale: Chemical Changes: Chemistry: GCSE (9:1)
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
Acid
A substance that increases the hydrogen ion concentration of a solution.
Base
A substance that reacts with an acid and neutralises it
Alkali
A soluble base, that produces OH- ions in solution
Types of chemicals that are bases
Metal oxides, metal hydroxides, metal carbonates, ammonia
Types of chemicals that are alkalis
Metal hydroxides, ammonia
Strong acids
Acids that fully ionise in water
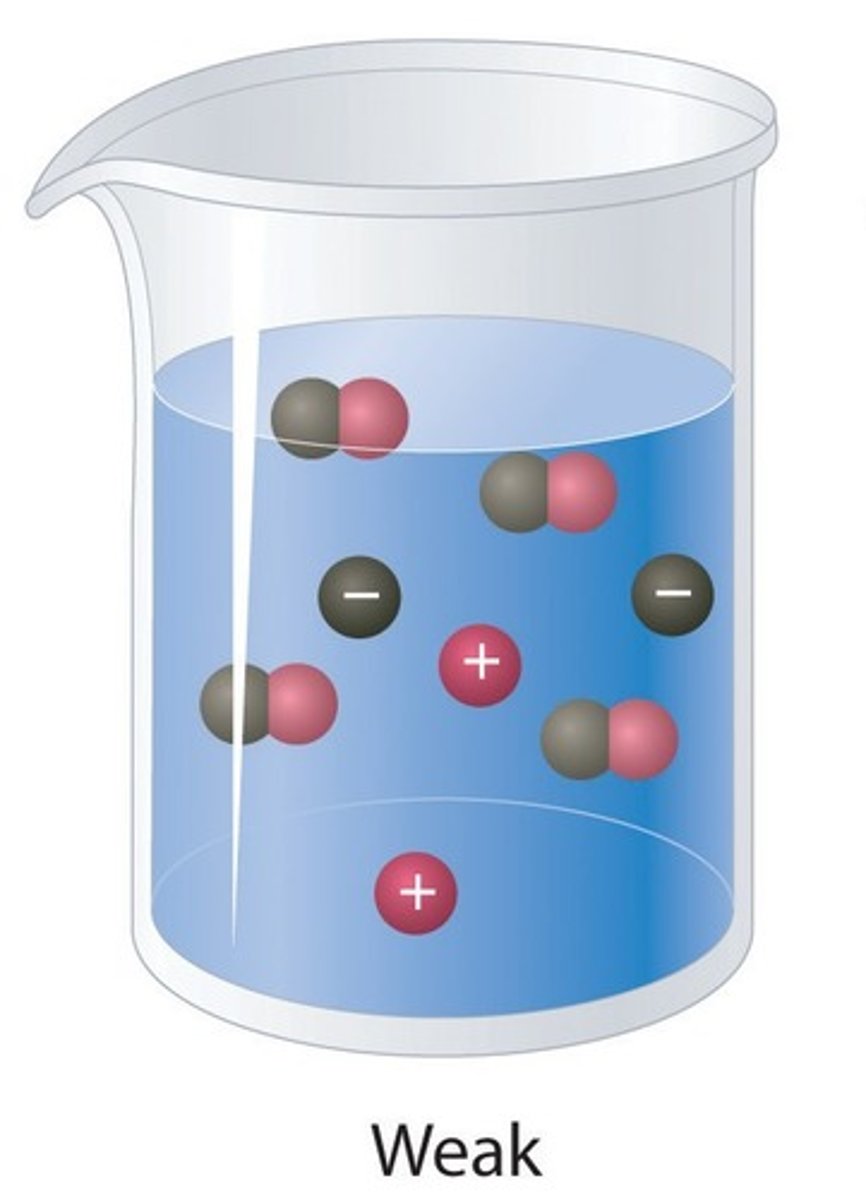
Weak acids
Acids that only slightly ionise in aqueous solution
Ionise
Split into ions
pH
A measure of H+ concentration
Ions produced by acids
H+
Ions produced by alkalis
OH-
Neutral pH
7
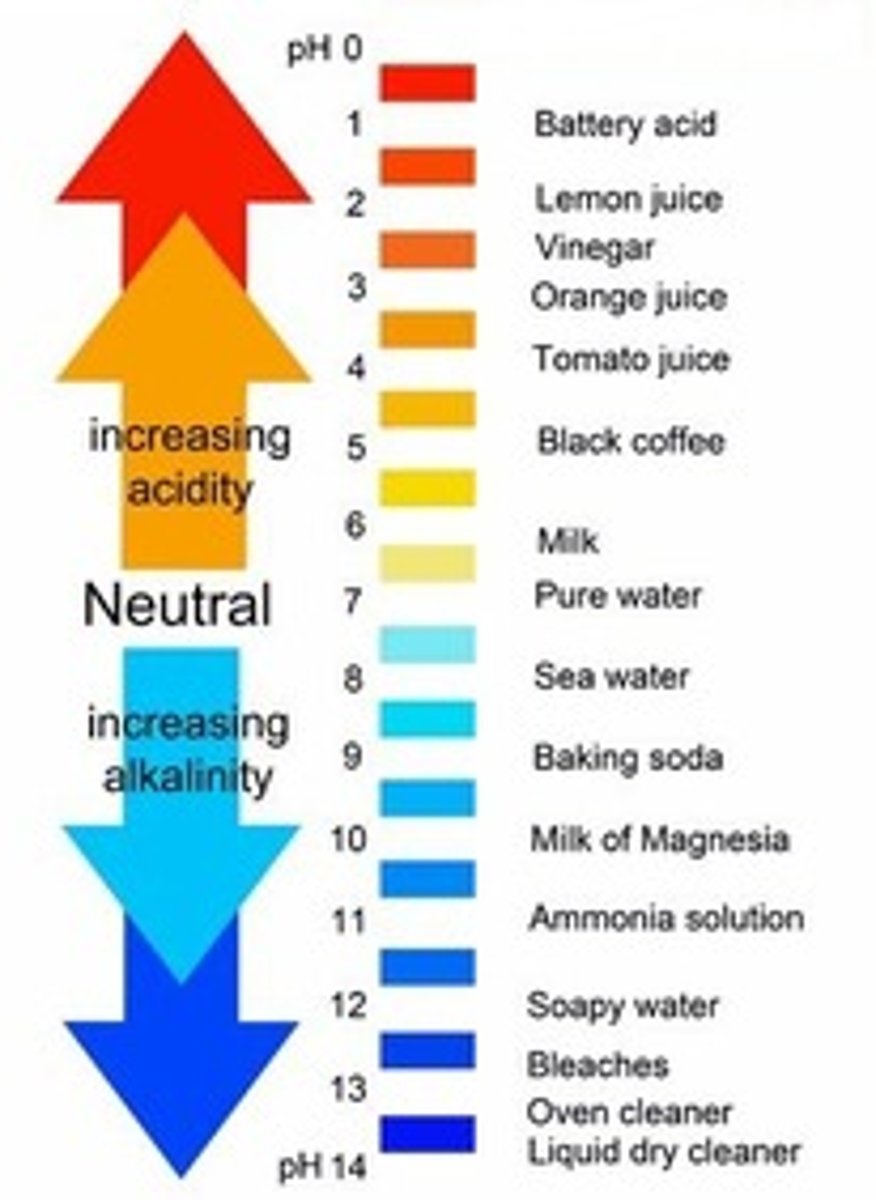
pH of acids
less than 7
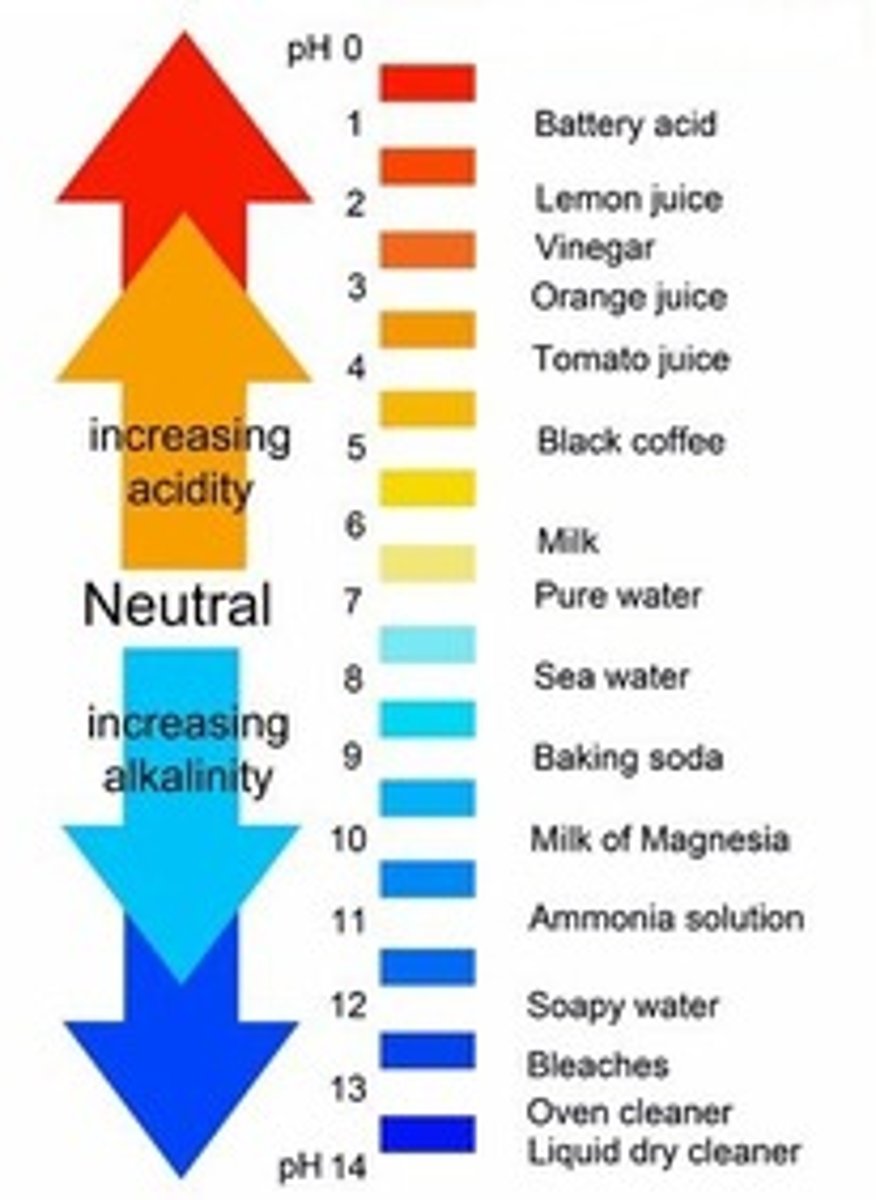
pH of alkalis
more than 7
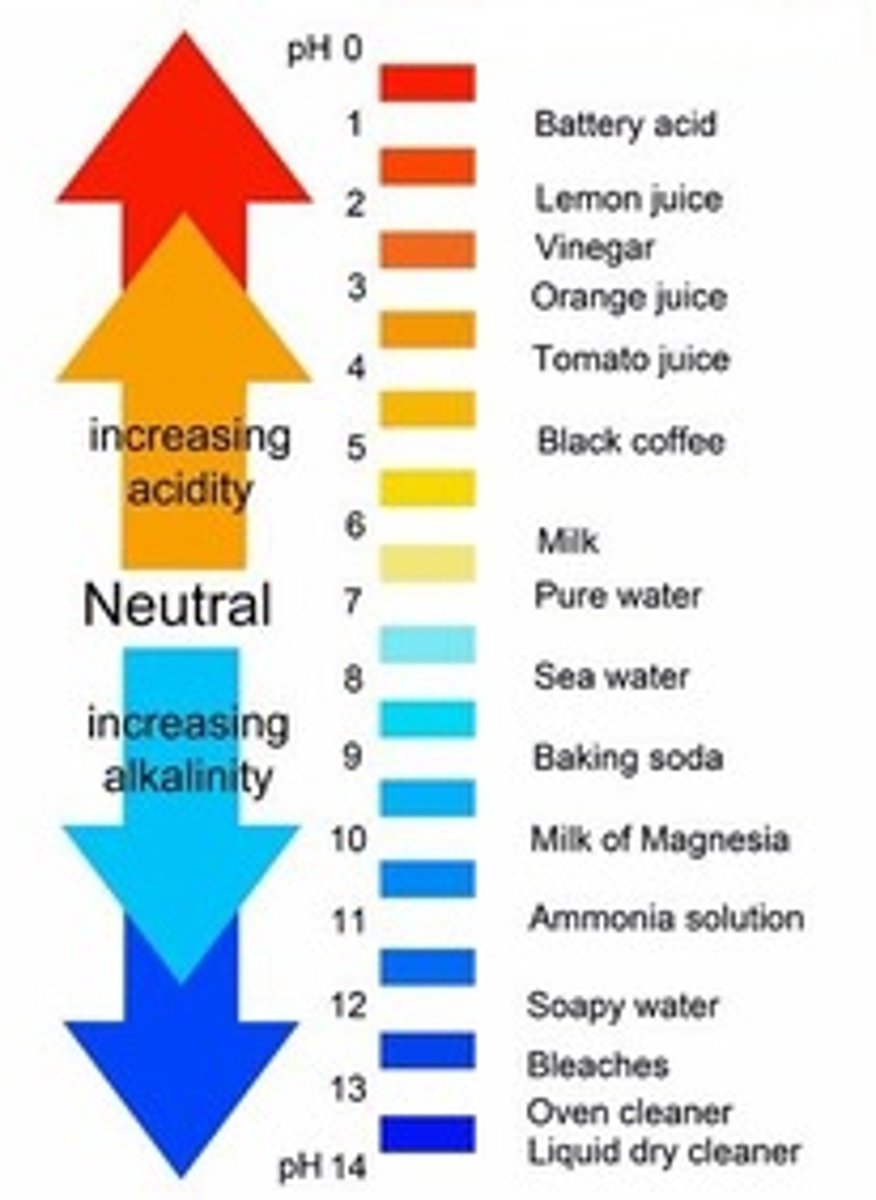
Neutralisation reaction
The reaction of an acid and a base forming a salt and water
Ionic equation for an acid and an alkali
H+(aq) + OH-(aq) --> H2O(l)
Universal indicator
An indicator with a different colour for each pH value.
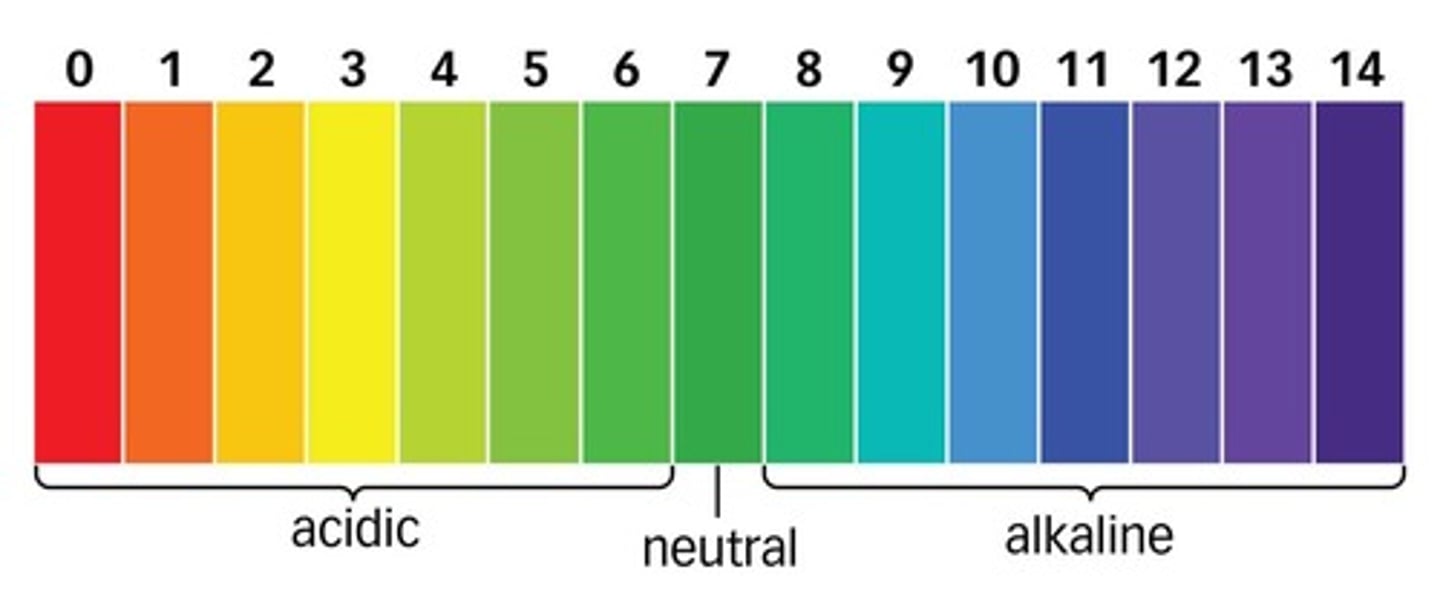
If pH decreases by 1
hydrogen ion concentration increases x10
At pH 7
concentration of H+ = concentration of OH-
Strong acid
An acid that ionises completely in water
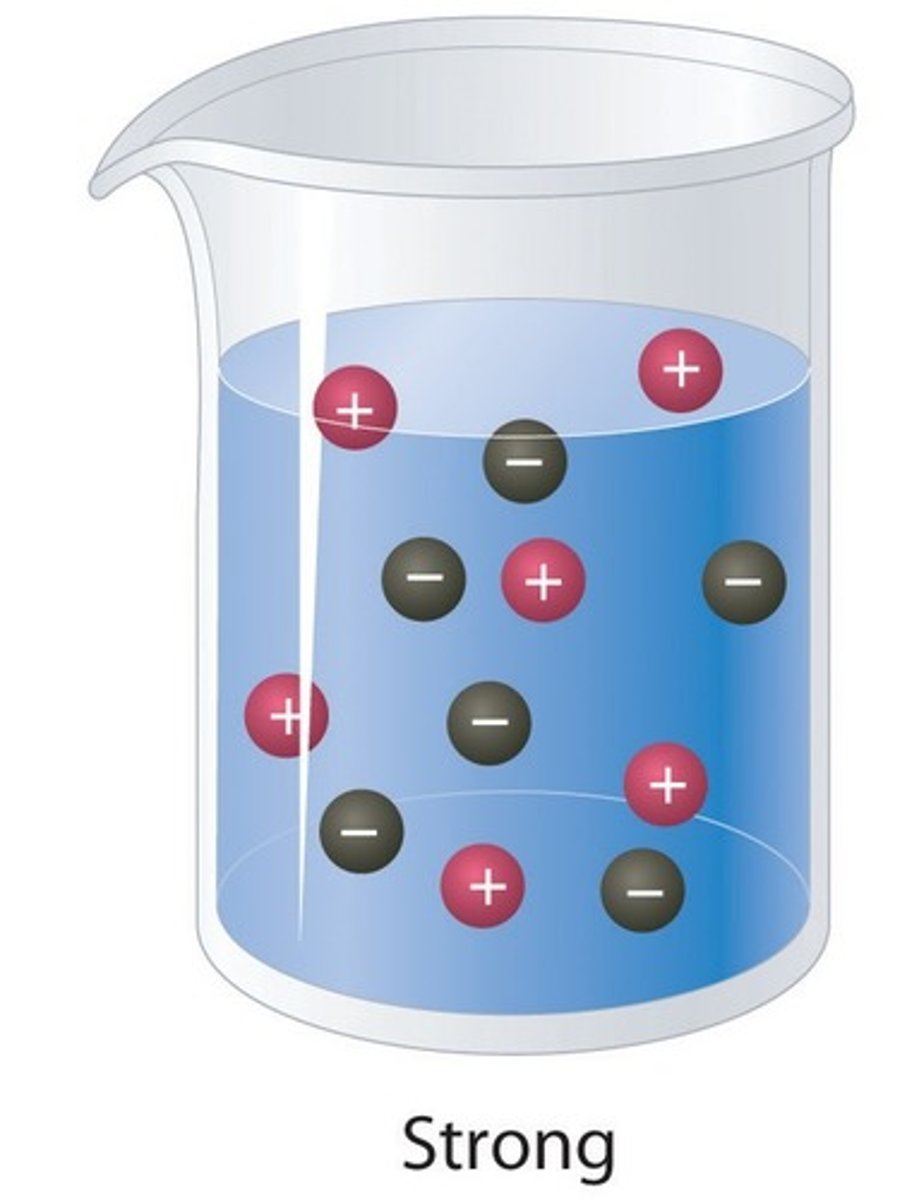
Weak acid
An acid that only partially ionises in water