10 Reaction rates and equilibrium
1/46
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
47 Terms
RoR def
how fast a reactant is used up/how fast a product is being formed
formula for RoR
RoR =
t=seconds
units of RoR
moldm-3s-1
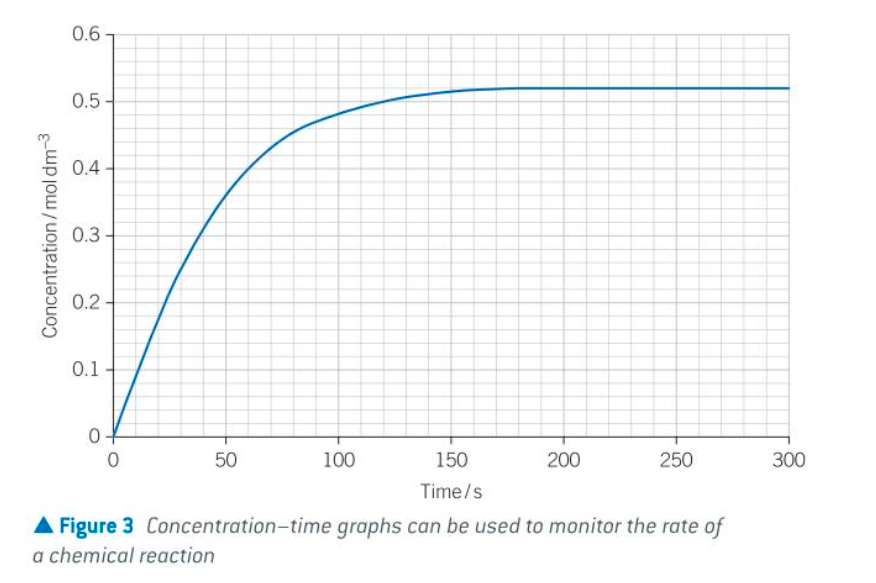
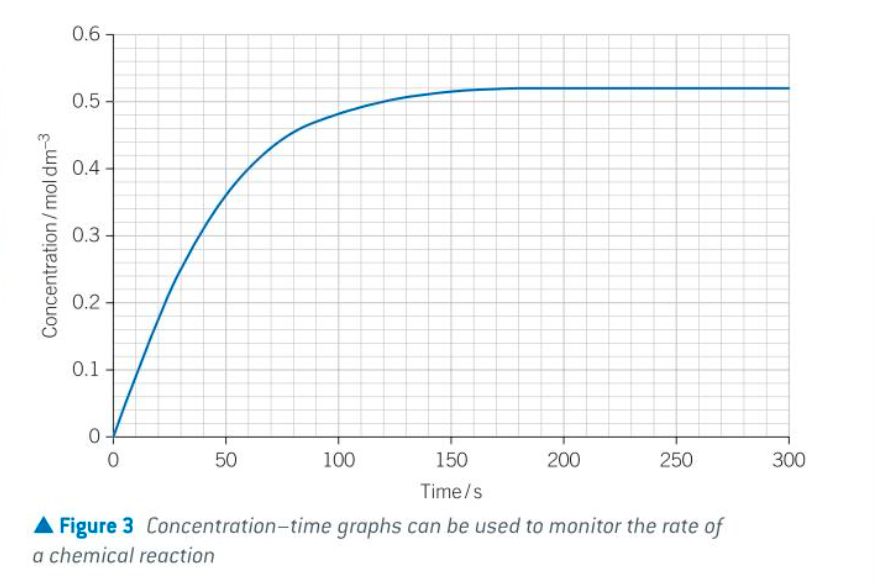
some things about RoR
RoR fastest at start of reaction as each reactant is in its highest concentration
the RoR slows down as reaction proceeds because reactants are being used up so concs decrease
once one of the reactants has been completely used up, the concs stop changing and the RoR is 0
factors that change RoR
conc (or pressure when reactants are gases)
temperature
use of a catalyst
SA of solid reactants
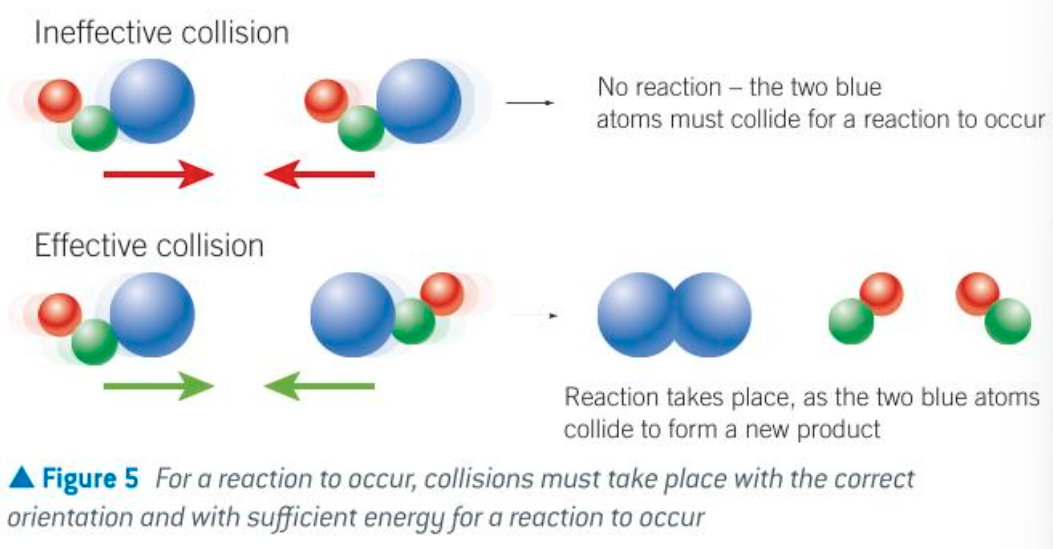
collision theory
two reacting particles must collide for a reaction to occur
what are the 2 conditions to an effective collision
particles collide w correct orientation
particles have sufficient energy to overcome the Ea barrier of the reaction
how does increasing conc affect RoR
increase in conc increases no. particles in same volume
particle r closer together and collide more frequently
in a given period of time there will therefore be more effective collisions (correct orientation and sufficient energy)
an increased RoR
how does increasing pressure of a gas affect RoR
increasing pressure compresses a gas into a smaller volume
conc of gas molecules increases as the same no. gas molecules occupy a smaller volume
gas molecules are closer together so collide more frequently
leading to more effective collisions in same time
methods to follow the progress of a reaction
monitoring the removal (decrease in conc) of a reactant
following the formation (increase in conc) of a product
methods to determine the RoR of a reaction that produces a gas
monitoring the volume of gas produced at regular time intervals using gas collection “continuous monitoring method”
monitoring the loss of mass of reactants using a balance
catalyst def
a substance that increases the rate of a chemical reaction without being used up in the process
provides an alternative route for the reaction with lower activation energy
what r the 2 types of catalysts called
homogeneous and heterogeneous
describe a homogeneous catalyst
a homogeneous catalyst has the same physical state as the reactants
it reacts with the reactants to form an intermediate
the intermediate then breaks down to give the product and regenerates the catalyst
examples of homogeneous catalysts
esterification
ozone depletion
Fe2+ catalysts
what is the catalyst in esterification
sulfuric acid
how is a catalyst used in esterification
sulfuric acid
the H+ ions form the intermediate
all reactants are aq
what is the catalyst in ozone depletion
the Cl• radicals
how is a catalyst used in ozone depletion
Catalyst generation (initiation):
Cl2 -> 2Cl•
Propagation:
O3 + Cl• -> ClO• (intermediate) + O2
ClO• + O3 -> Cl• + 2O2
the reactant (O3) and the catalyst (Cl•) are both gases
overal rxn: 2O3 → 3O2
heterogeneous catalysts
a heterogeneous catalyst has a different physical state from the reactants
usually solids
how do heterogeneous catalysts work
reactant molecules are absorbed (weakly bonded) onto the surface of the catalyst where the reaction takes place
after reaction, the product molecules leave the surface of the catalyst by desorption
industrial processes that use heterogeneous catalysis
making ammonia (Haber process)
reforming
hydrogenation of alkenes
making sulfur trioxide for sulfuric acid (contact process)
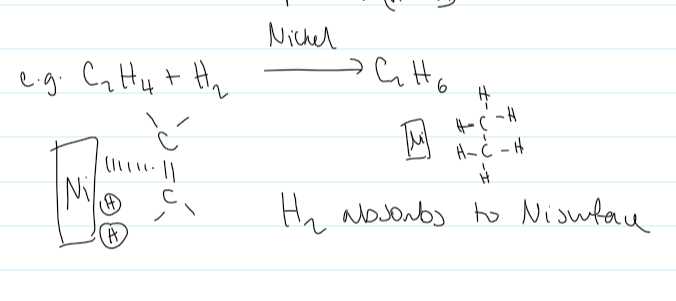
how is a catalyst used in hydrogenation of alkenes
nickel
the H2 absorbs to the Ni surface
catalyst for making ammonia/Haber process
Fe(s)
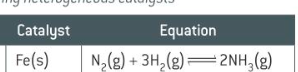
catalyst and equation for reforming

catalyst for hydrogenation of alkenes
Nickel
catalyst and equation for making sulfur trioxide for sulfuric acid
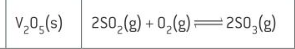
why is using catalysts more sustainable
catalysts increase the RoR of many industrial processes by lowering the Ea
this then reduces the temp needed for the process and the energy requirements
therefore less electricity/fossil fuels used
product made faster w less energy - cuts costs
what do catalytic convertors contain
a catalyst made of platinum, rhodium and palladium
why are catalytic convertors used
the hot exhaust gases that pass over the catalyst ensures harmful gases are converted into less harmful products
what is the Boltzmann distribution
the spread of molecular energies in gases
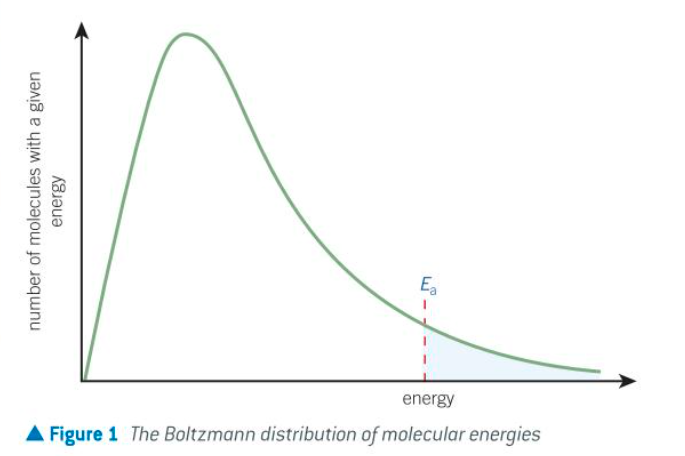
features of the Boltzmann distribution
no molecules have zero energy - the curve starts at the origin
the area under the curve is equal to the total number of molecules
there is no maximum energy for a molecule - the curve never meets the x-axis (energy)
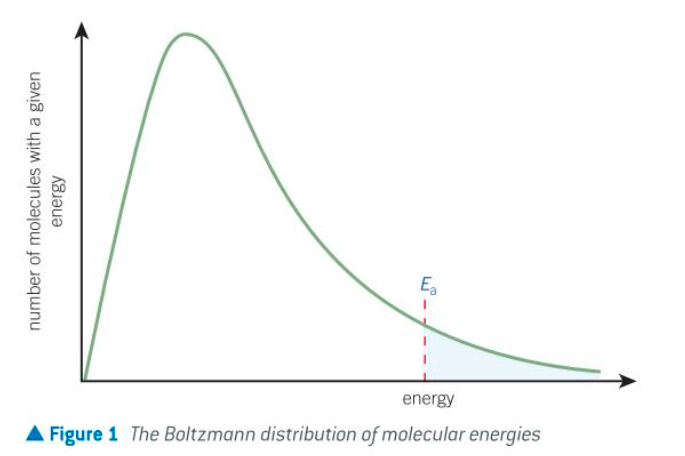
what is the y and x axis for a boltzmann distribution
y axis = number of molecules w a given energy
x axis = energy
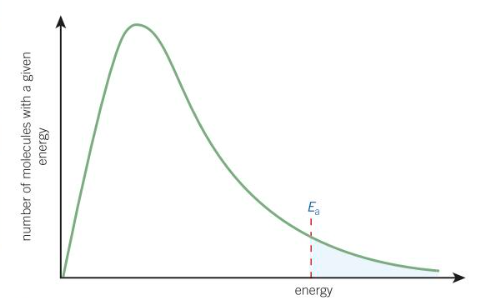
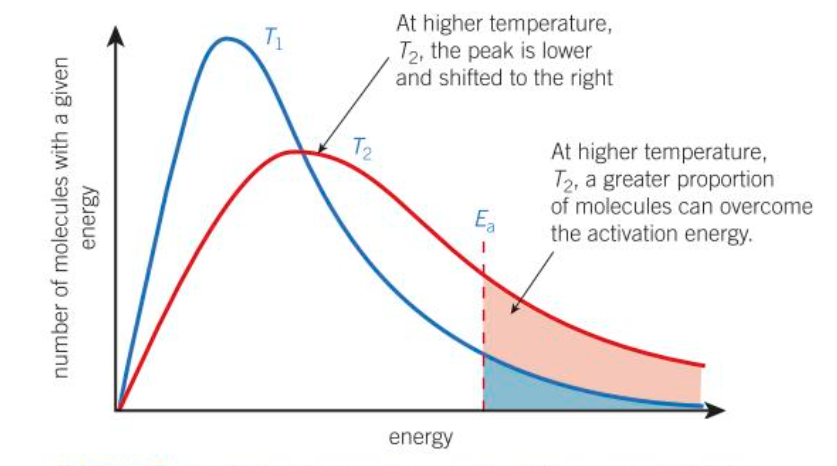
effect of temp on boltzmann distribution (think of graph)
as temp increases the avg energy of molecules also increases
a small proportion of molecules will still have low energy, but more molecules have higher energy
graph is now stretched over a greater range of energy values
the peak of the graph is lower on the y-axis and further along the x-axis: is at a higher energy
number of molecules is the same therefore area under curve is same
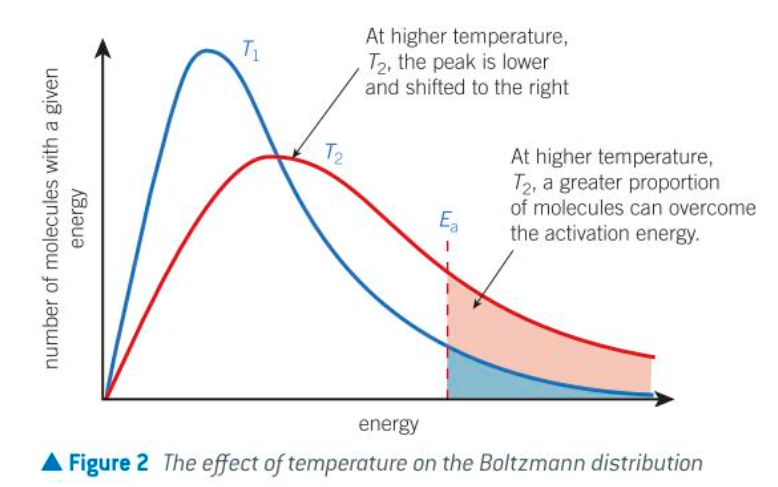
(relation to boltzmann distribution) at higher temp:…
more molecules have an energy greater than or equal to the Ea
therefore a greater proportion of collision will lead to a reaction, increasing the RoR
collisions will also be more frequent as the molecules are moving faster, but the increased energy of the molecules is much more important than the increased frequency of collisions
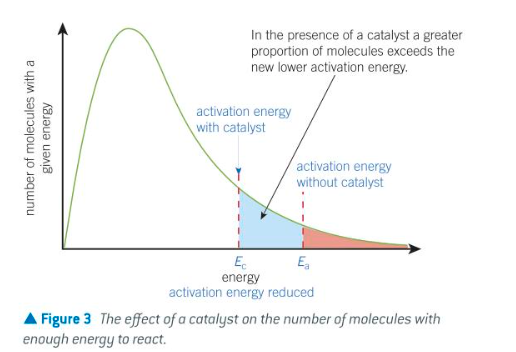
boltzmann distribution w catalyst graph
what does the position of equilibrium indicate
the position of equilibrium indicates the extent of the reaction
in a reversible reaction, if the temperature, pressure (gases), of concentration of the reactants or products is changed, then the position of equilibrium may change
le Chatelier’s principle
states that when a system in equilibrium is subjected to an external change, the system readjusts itself to minimise the effect of that change
effect of [] change on equilibrium
will change the rate of the forward or reverse reactions
position of equilibrium will change
increase in [] = shifts to RHS
decrease in [] = shifts to LHS
effect of temp change on equilibrium
direction equilibrium shifts to depends on sign of ΔH
increase in T = shifts to endothermic direction (ΔH is positive)
decrease in T = shifts to exothermic direction (ΔH is negative)
effect of pressure change on equilibrium
shifts to side that will reduce the pressure of the system
increase in pressure = shifts to side w fewer moles of gas
decrease in pressure = shifts to side w most moles of gas
effect of catalyst on equilibrium
doesn’t change position
only speeds up the rate of forward and reverse rxns equally
will increase the rate at which an equilibrium is established
equilibrium law
equilibrium law defines the equilibrium constant Kc in terms of concentrations

requirements for a dynamic equilibrium to be established
closed system
concentrations are constant
rate of forward reaction = rate of reverse reaction
diagram of boltzmann distribution when using a catalyst + explanation
catalyst allows rxn to proceed via a different route with a lower Ea
therefore there are more molecules with energy above Ea with a catalyst
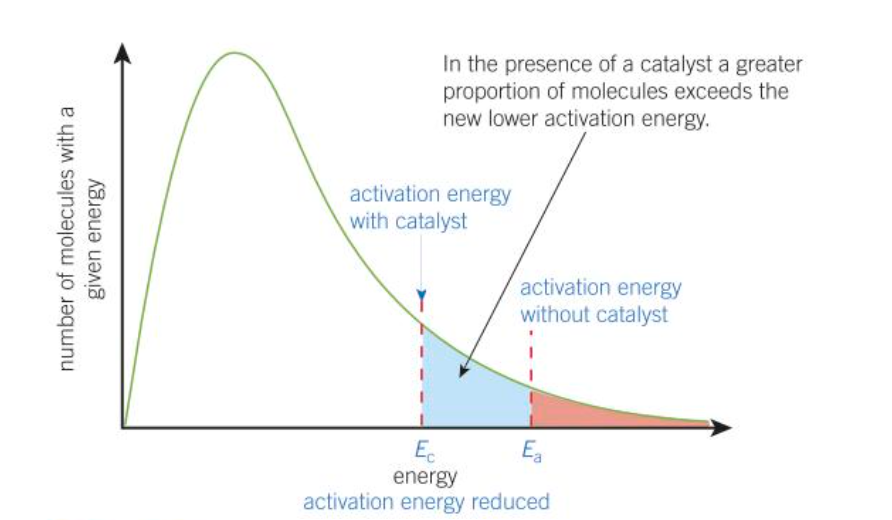
effect of a higher temp on rxn - boltzmann distribution
if given the value of the Ea and ΔH for the forward reactions, what is the Ea for the reverse reaction?
ΔH + Ea
be careful of signs