Day 5 - Bioenergetics and Oxidative Metabolism
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
We breathe electron ________. We eat electron ________.
acceptors
donors
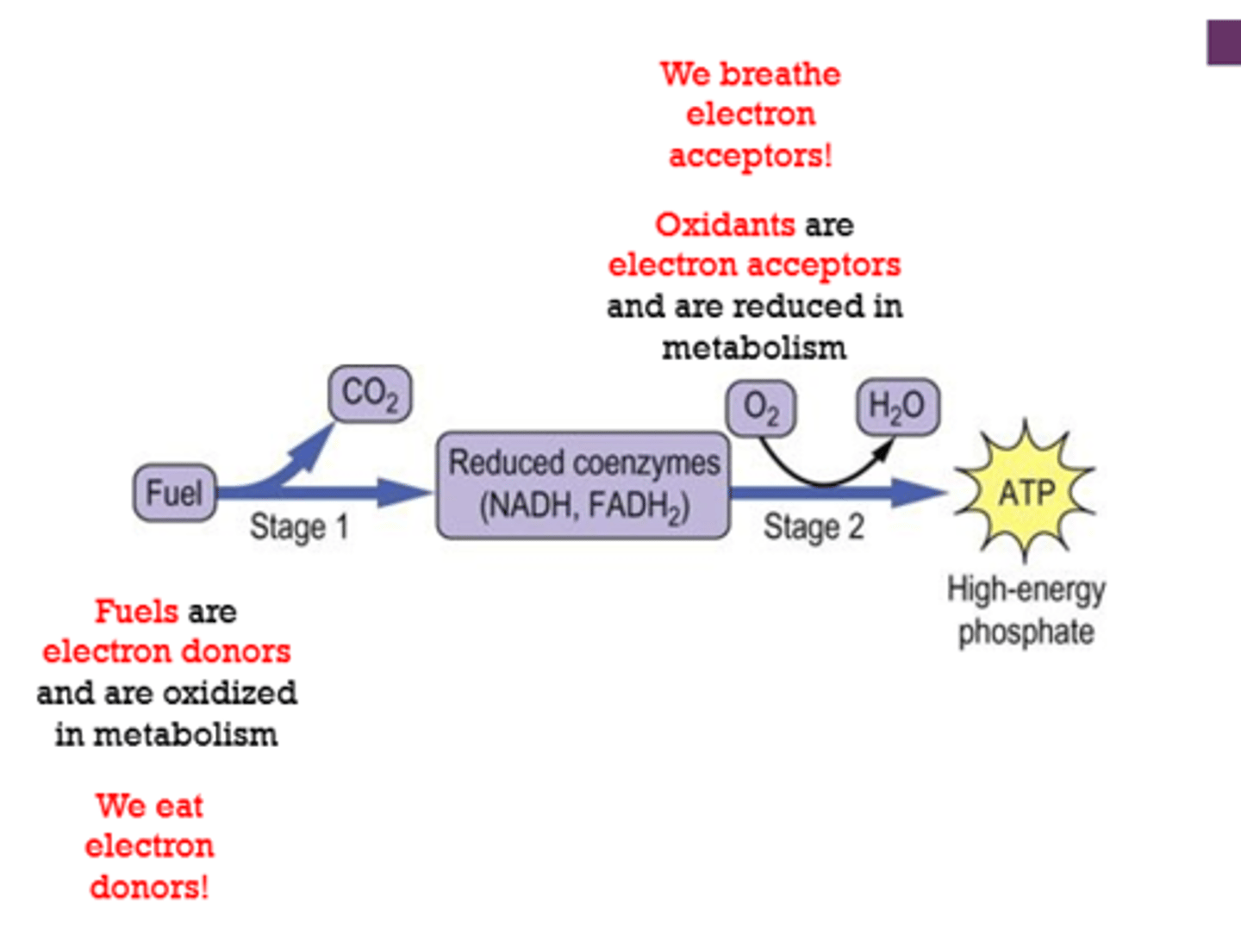
Fuels are electron [donors/acceptors] and are [reduced/oxidized] in metabolism.
Oxidants are electron [donors/acceptors] and are [reduced/oxidized] in metabolism.
donors
oxidized
acceptors
reduced
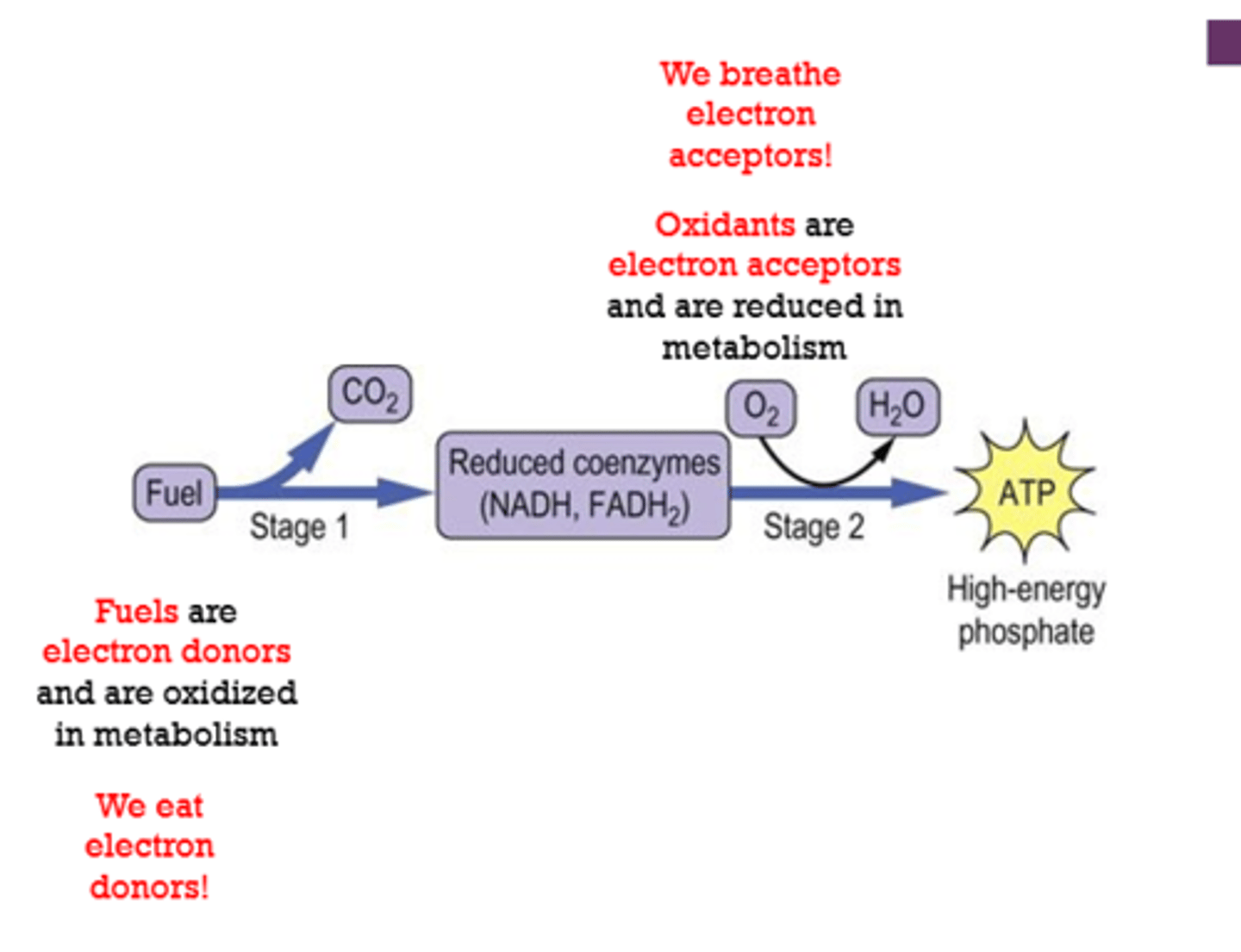
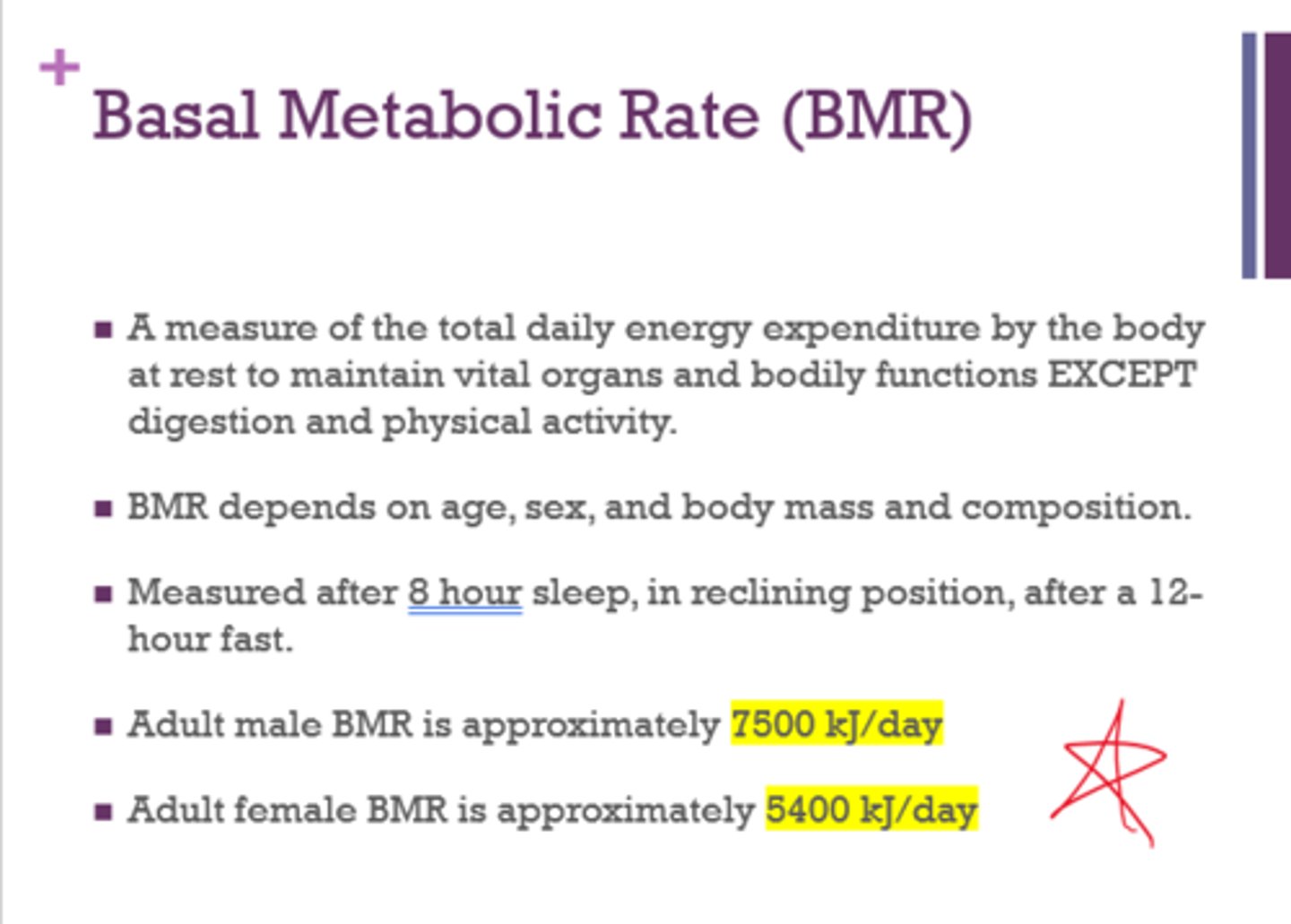
What is basal metabolic rate (BMR) a measure of?
What 2 bodily functions are an exception to BMR?
A measure of the total daily energy expenditure by the body at rest to maintain vital organs and bodily functions
EXCEPT digestion and physical activity
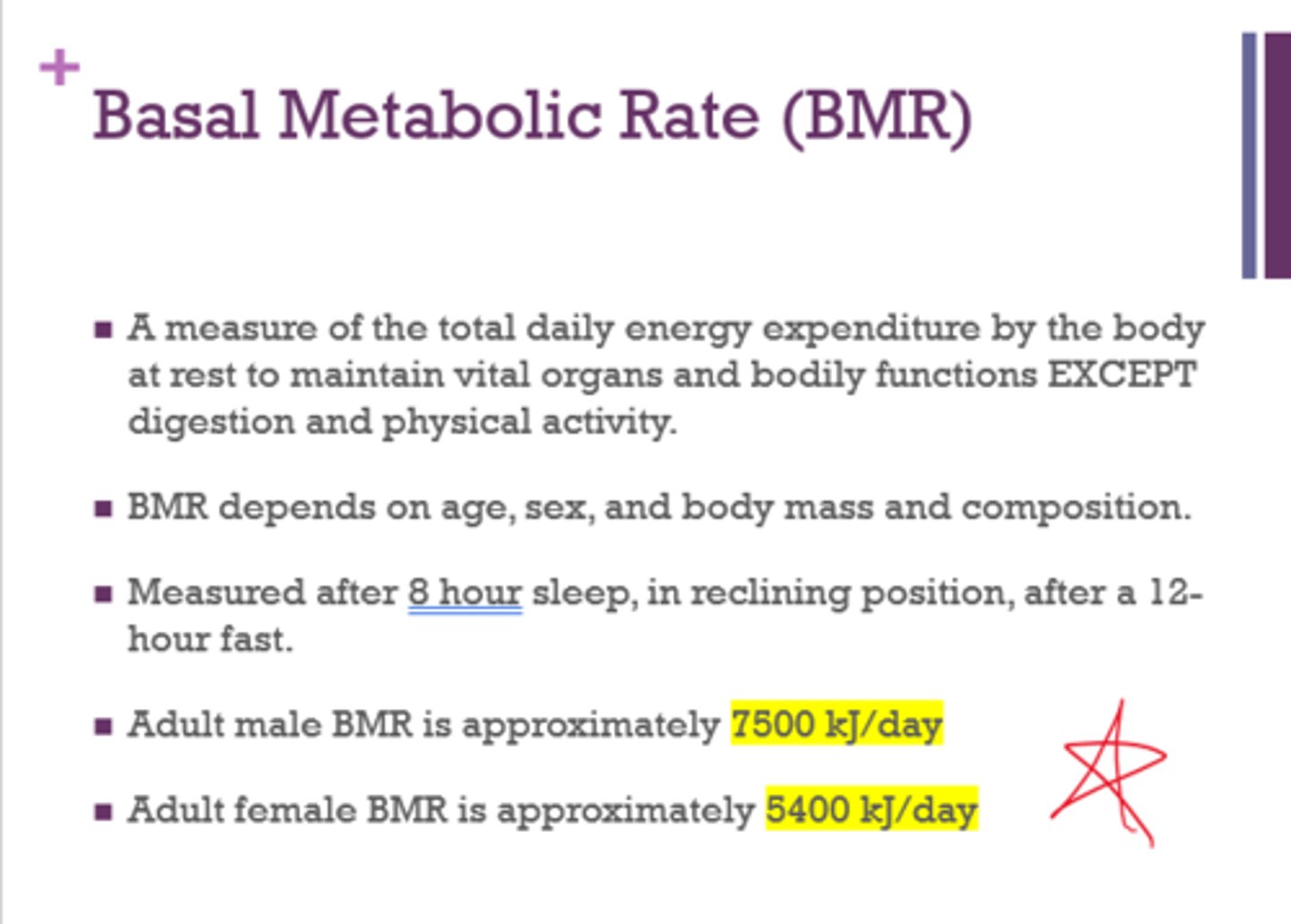
What is the average adult male and female BMR?
(for sure on test - will be given a range and need to see if it's male or female that falls within that range)
male - 7500 kJ/day
female - 5400 kJ/day
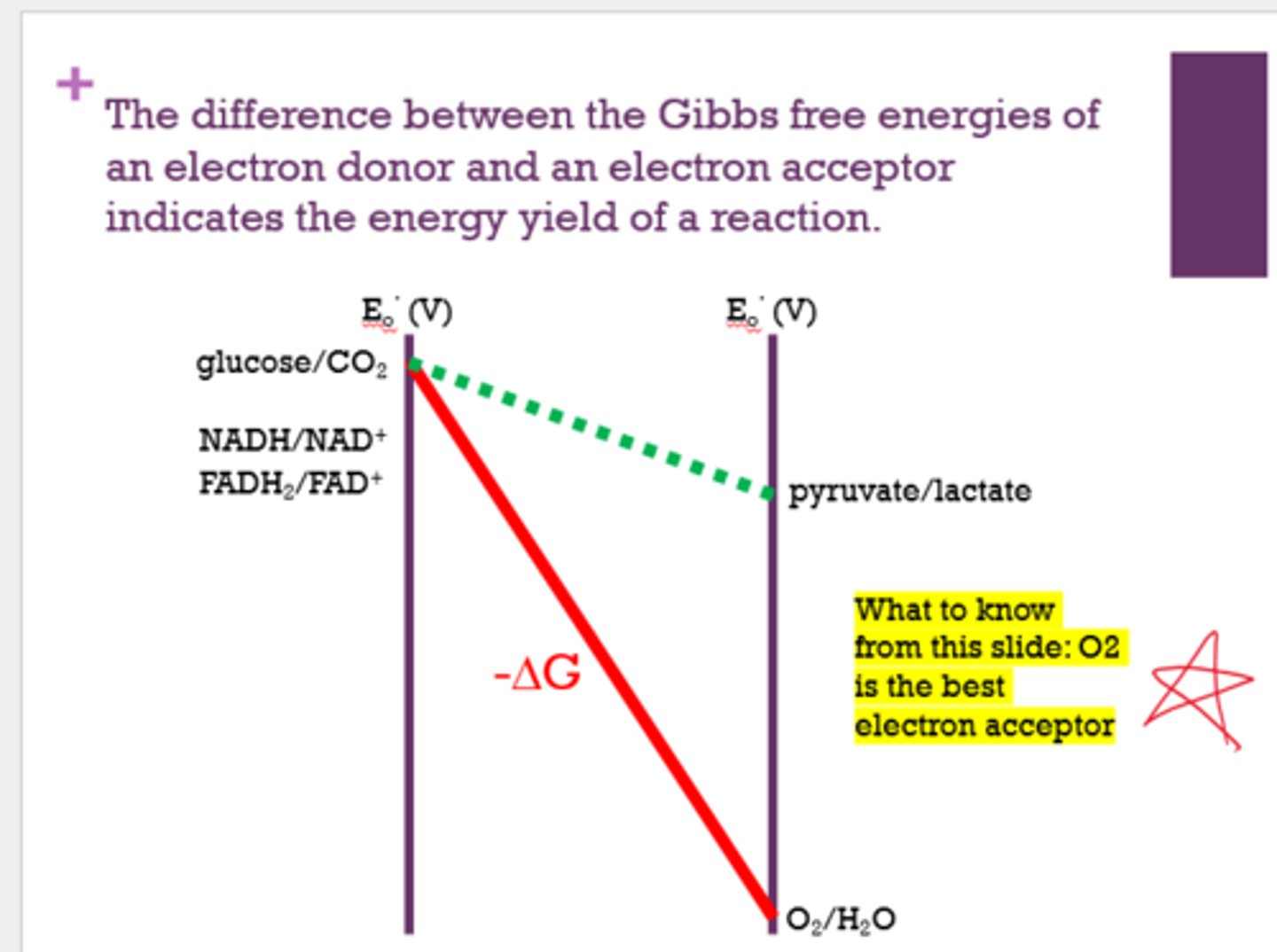
What is the best electron acceptor?
Oxygen
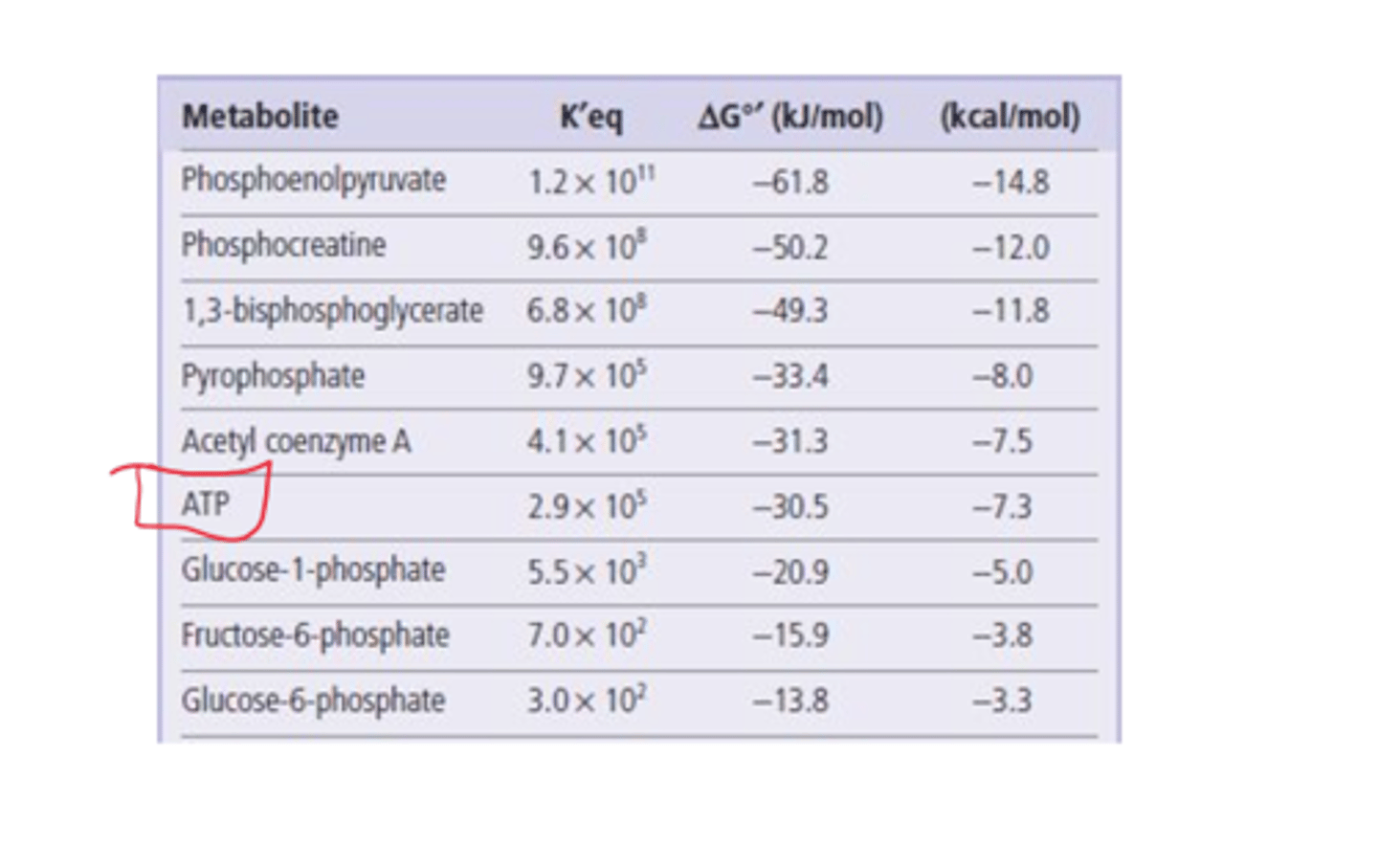
What is delta G for ATP?
-30.5 kJ/mol
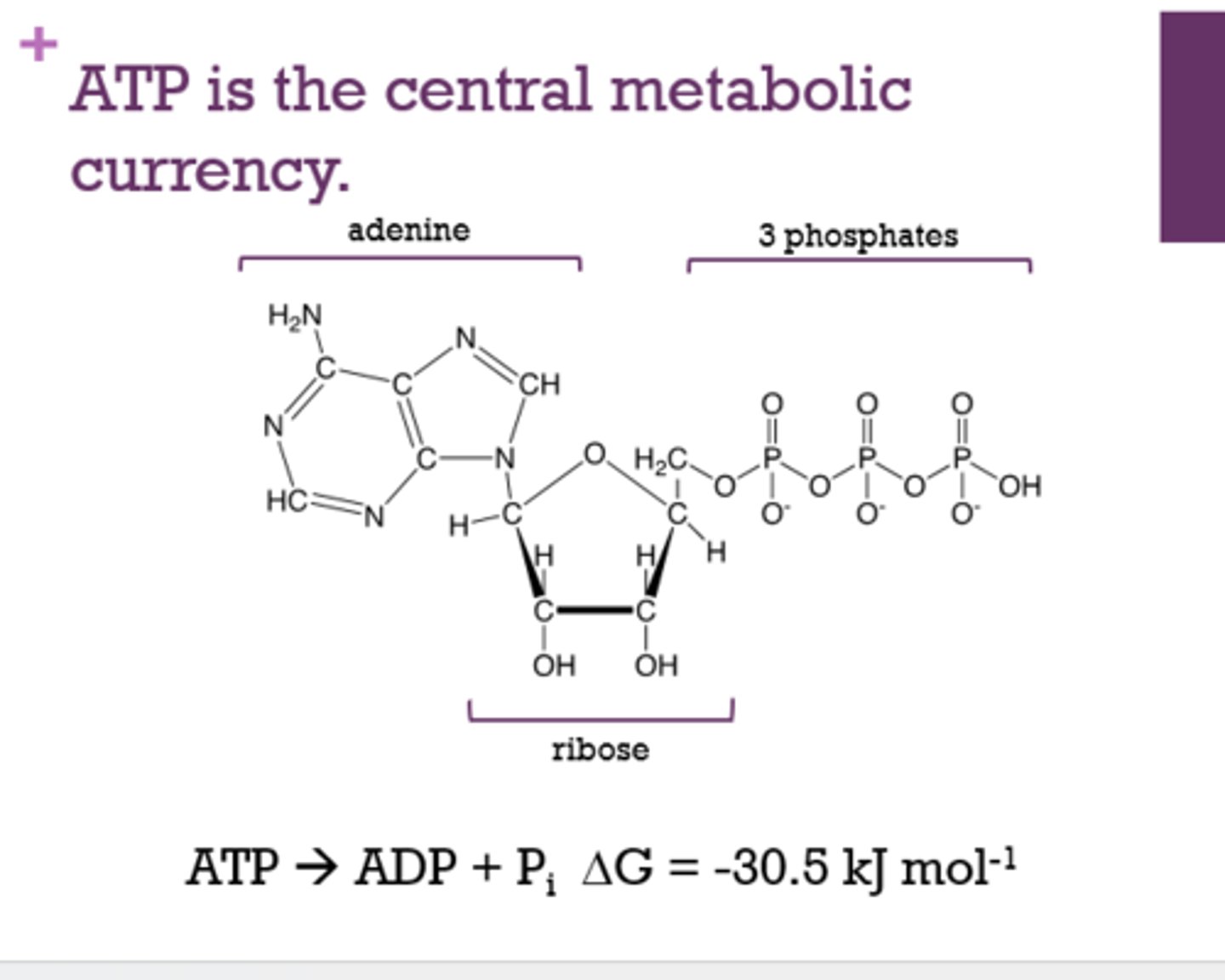
What is the central metabolic currency?
ATP
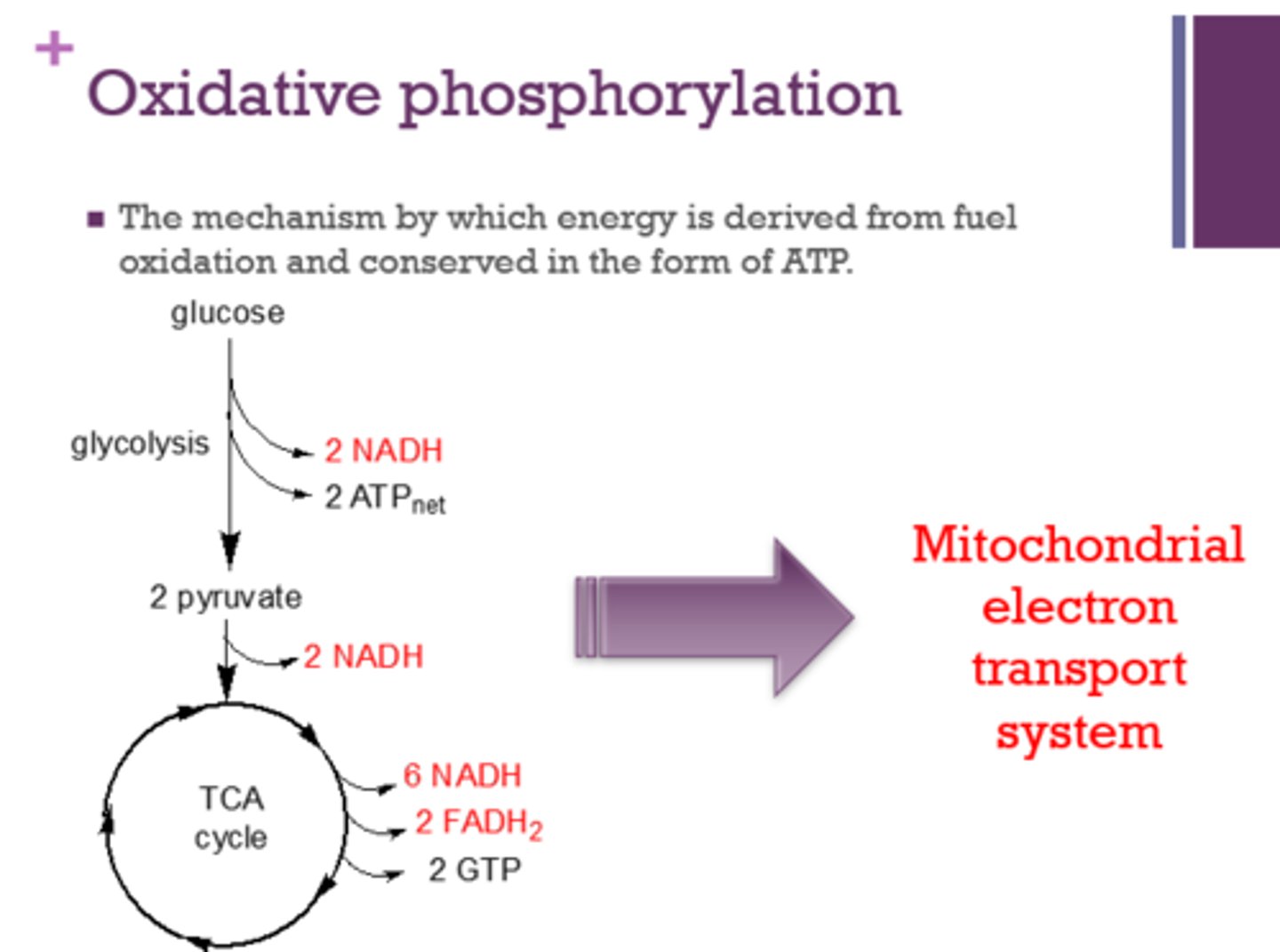
Oxidative phosphorylation is the mechanism by which energy is derived from _____ and conserved in the form of _____.
fuel oxidation
ATP
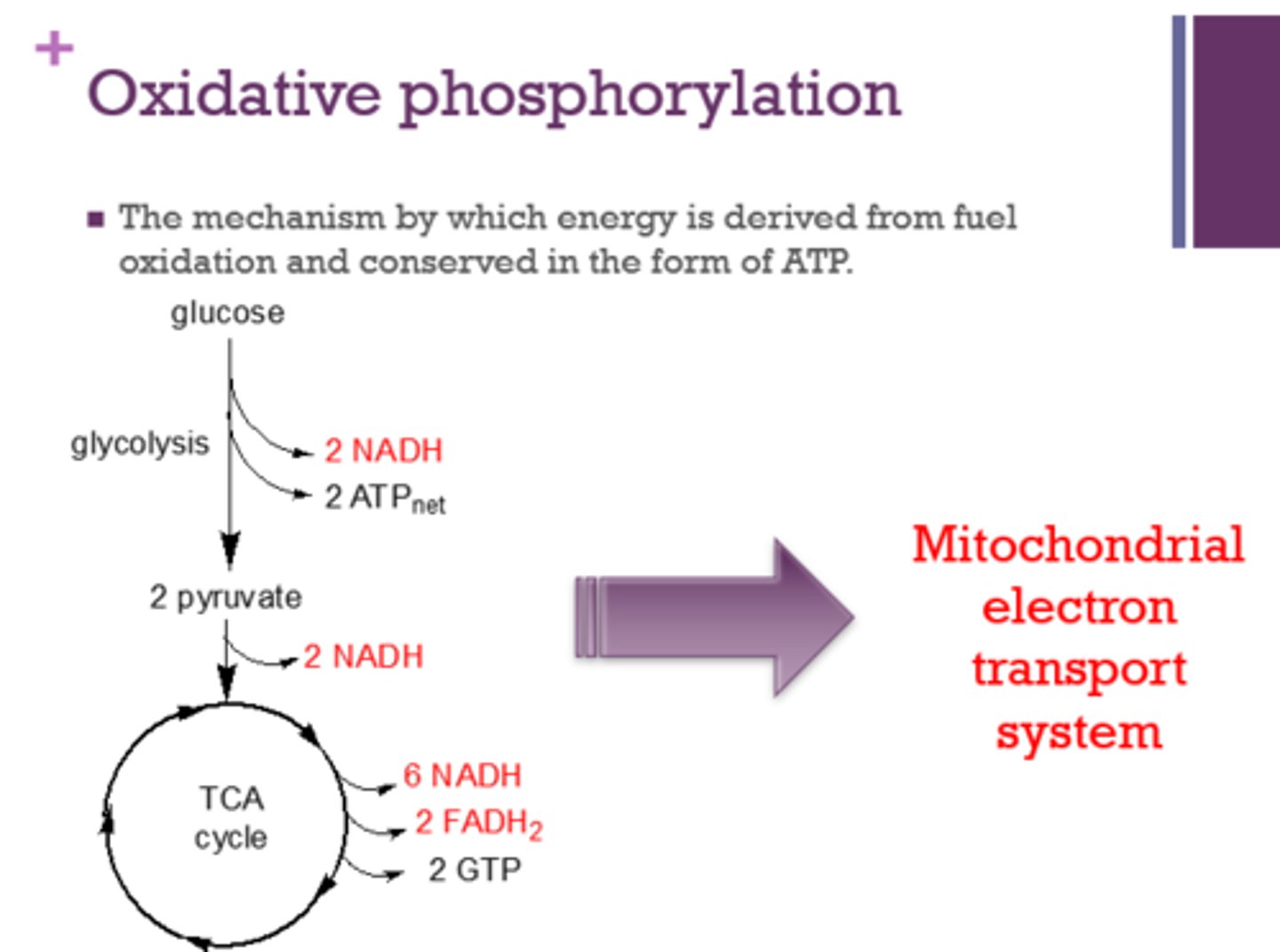
What system is involved in oxidative phosphorylation?
mitochondrial electron transport system
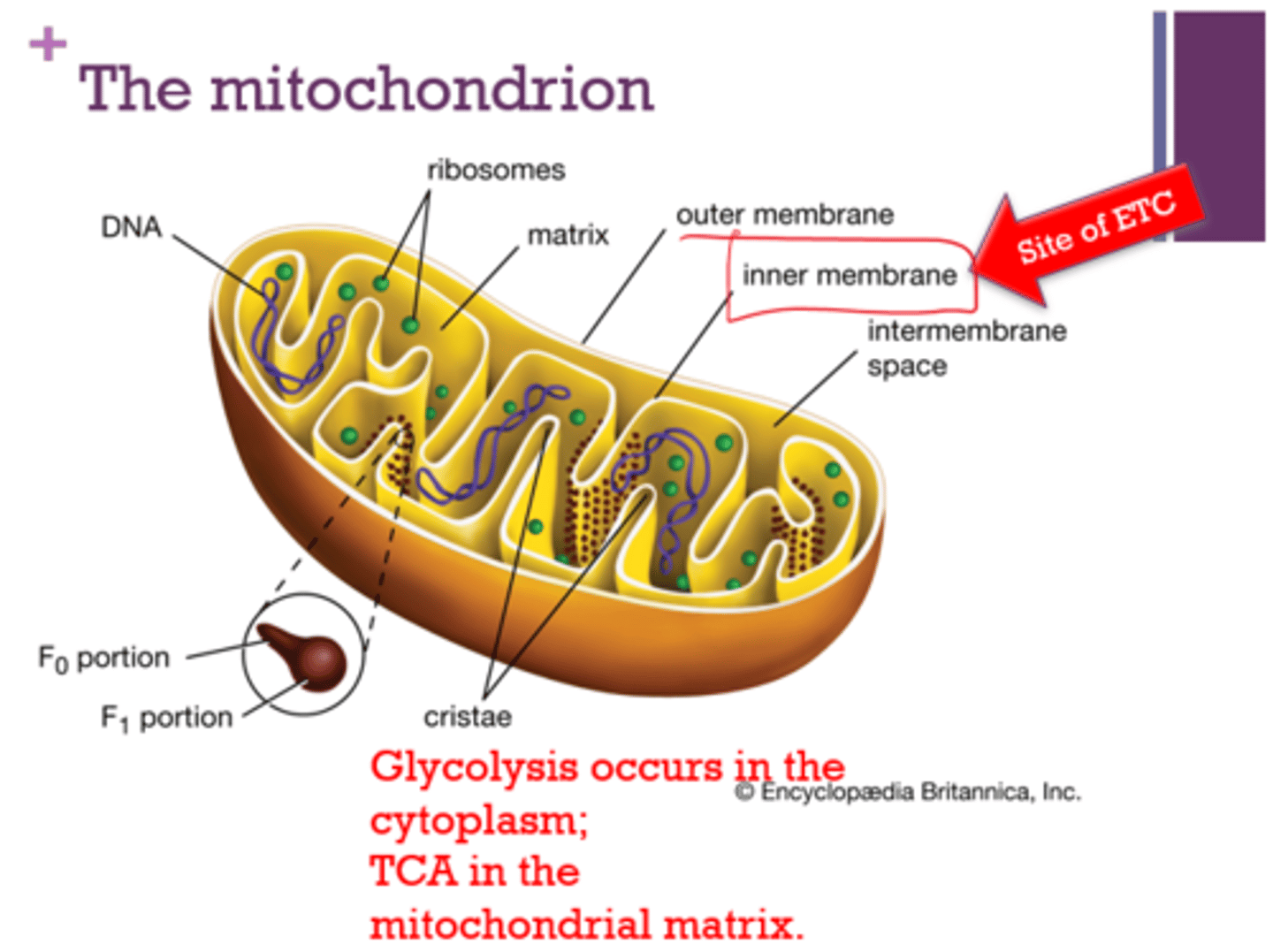
Where is the site of ETC (electron transport chain)?
inner mitochondrial membrane
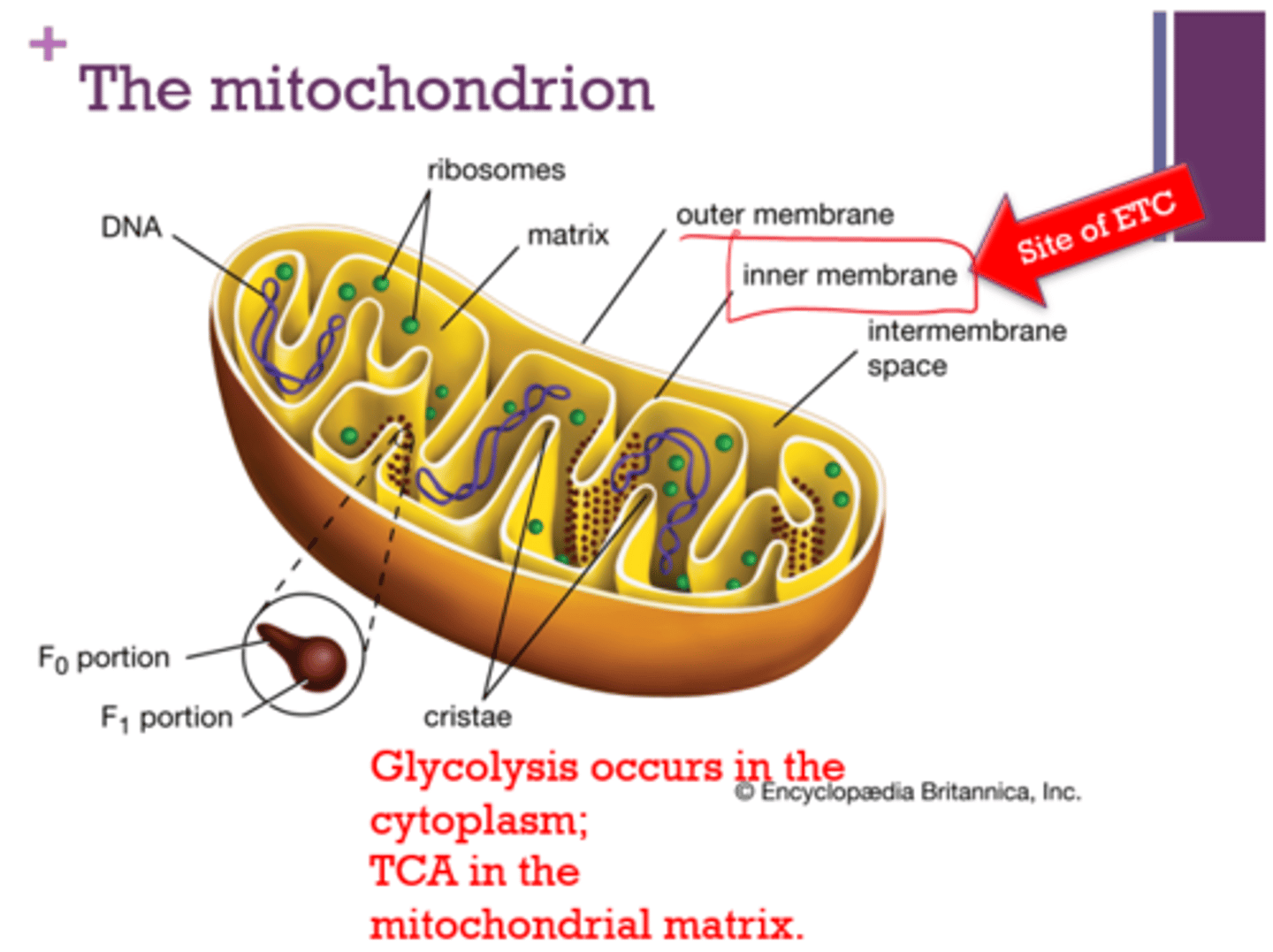
Where does glycolysis occur?
Where does TCA occur?
- cytoplasm
- mitochondrial matrix
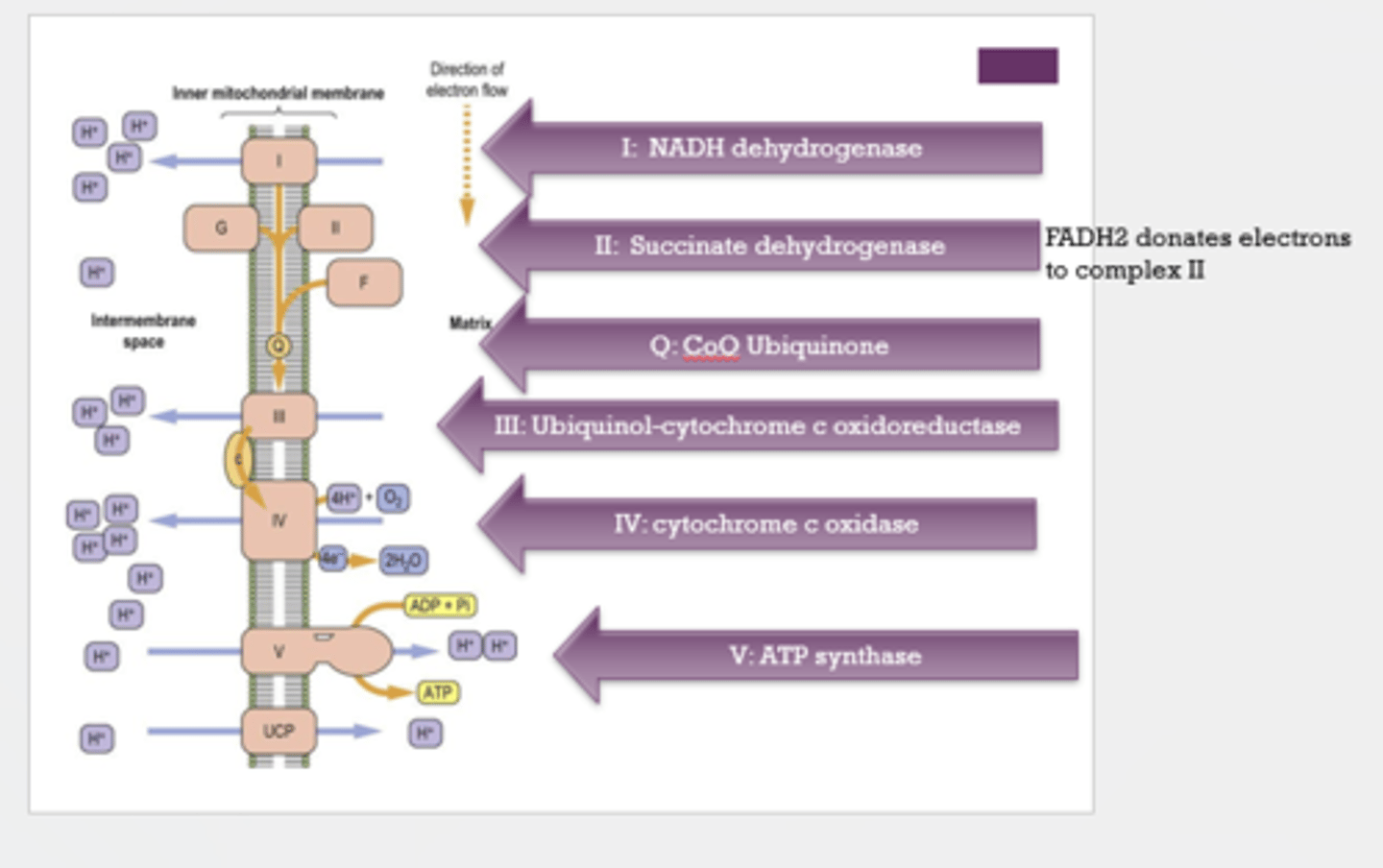
List in order the 6 components of the electron transport chain.
- Complex I: NADH dehydrogenase
- Complex II: succinate dehydrogenase
- Q: CoQ Ubiquinone
- Complex III: Ubiquinol-cytochrome c oxidoreductase
- Complex IV: cytochrome c oxidase
- Complex V: ATP synthase
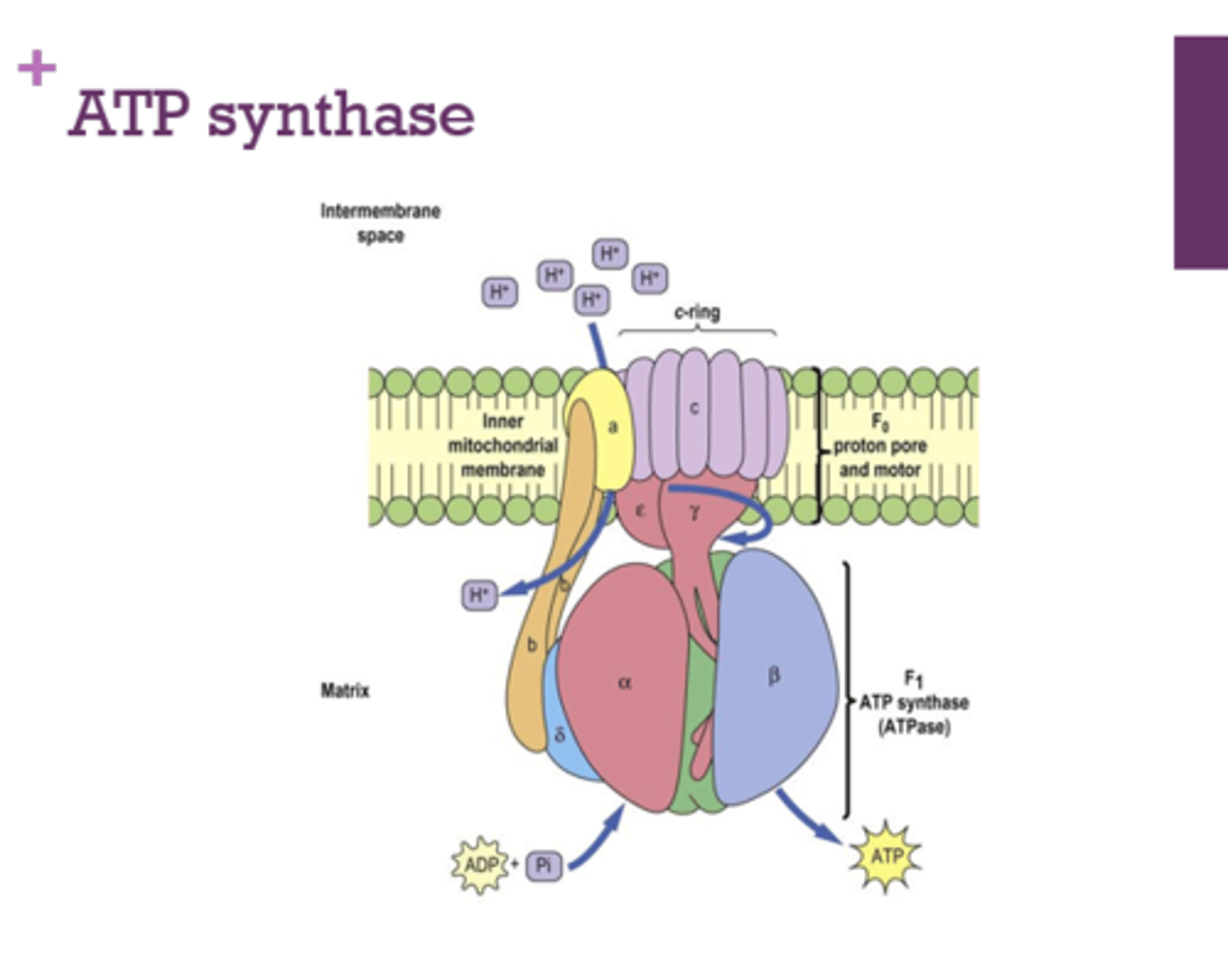
What membrane is ATP synthase embedded in?
inner mitochondrial membrane
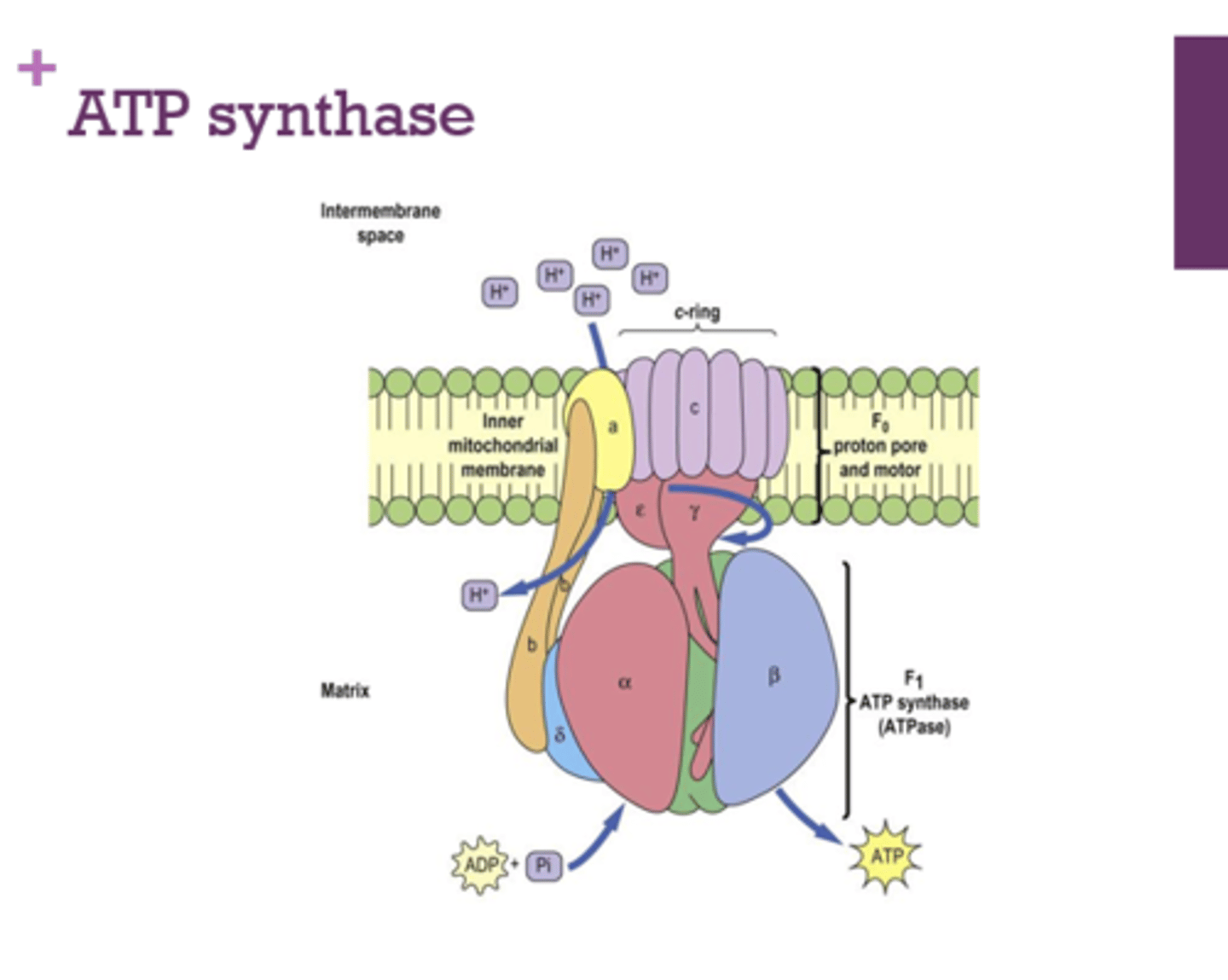
Before going through ATP synthase, where are the protons that make up the proton motive force?
intermembrane space
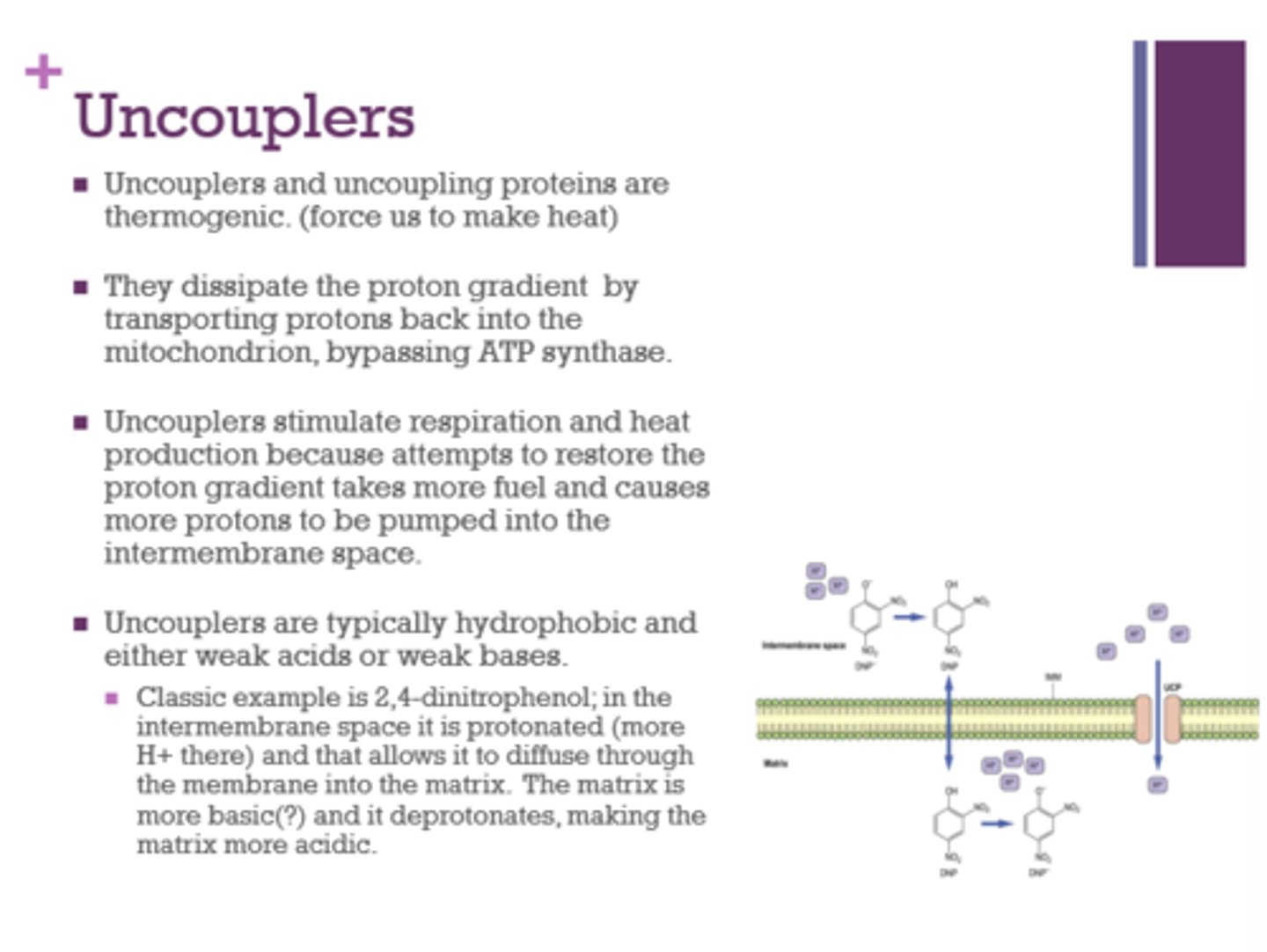
What are 2 characteristics of uncouplers?
Give an example.
- hydrophobic, weak acids or weak bases
- 2,4-dinitrophenol
State where each uncoupling proteins are found in the body:
1. UCP1:
2. UCP2: (for this one, state what 2 diseases its associated with)
3. UCP3:
4. UCP 4 and 5:
1. brown adipose tissue
2. obesity and type II diabetes
3. skeletal muscle
4. brain

Why are uncoupling proteins thermogenic?
creates a gradient that drives respiration and makes it thermogenic? (double check)
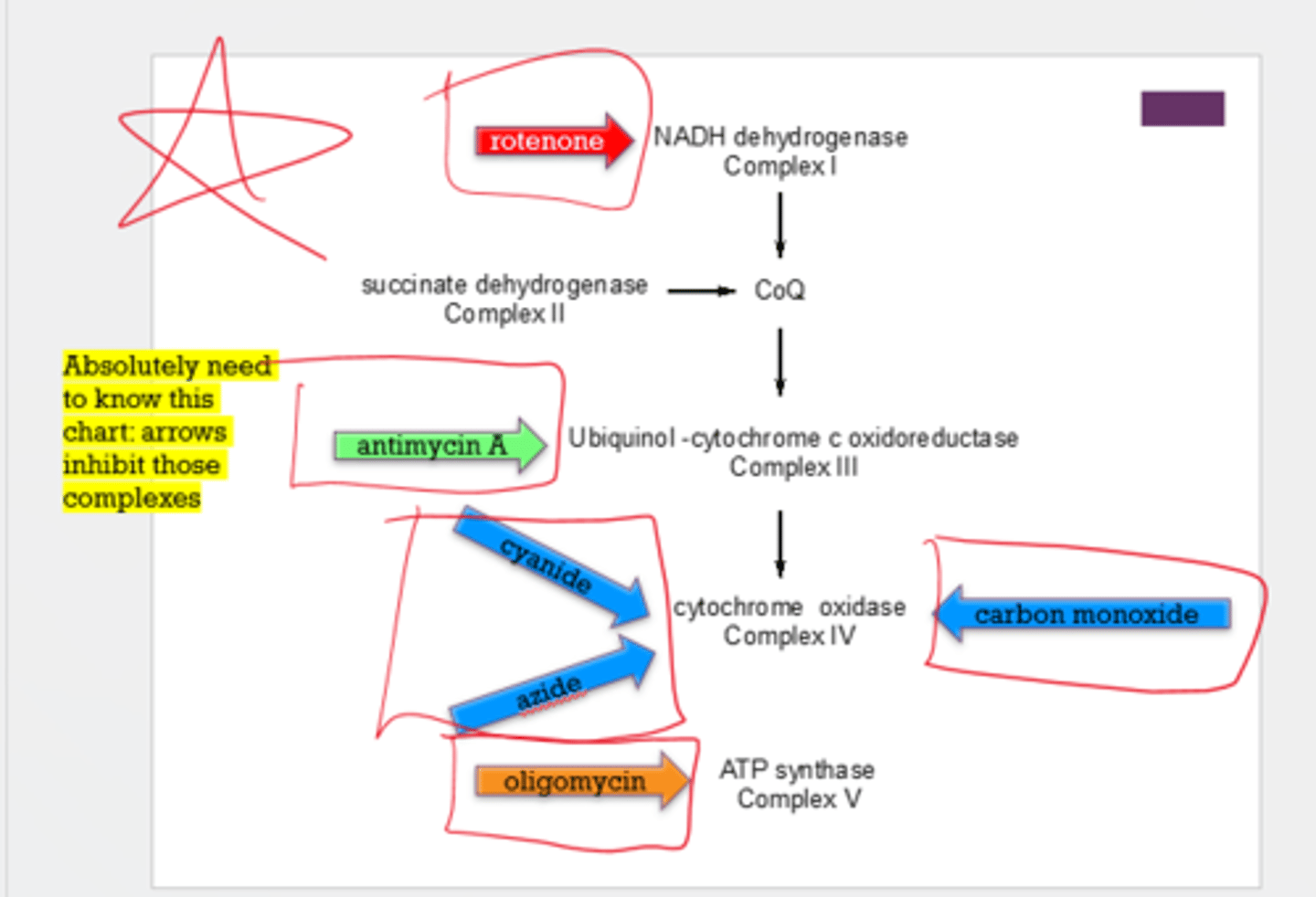
Name what inhibits each complex:
1. Complex I
2. Complex II
3. Complex III
4. Complex IV
5. Complex V
1. rotenone
2. n/a
3. antimycin A
4. carbon monoxide, cyanide, azide
5. oligomycin
Note: he said to create a table to know where these are and what they inhibit. There is for sure 1 question from this chart on the test.
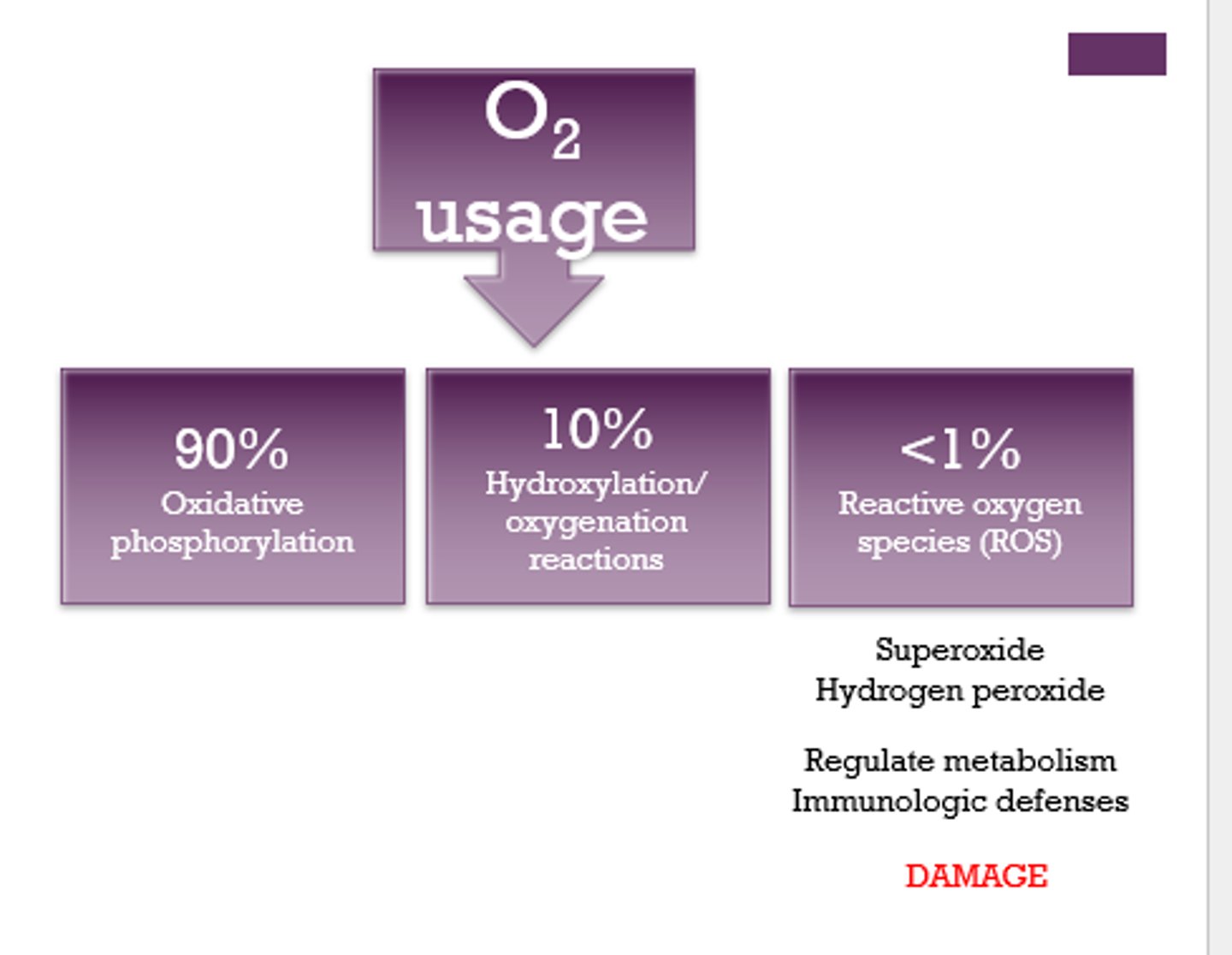
State the % of O2 that is used by each reaction:
1. oxidative phosphorylation:
2. hydroxylation/oxygenation reactions:
3. reactive oxygen species (ROS):
1. 90%
2. 10%
3. less than 1%
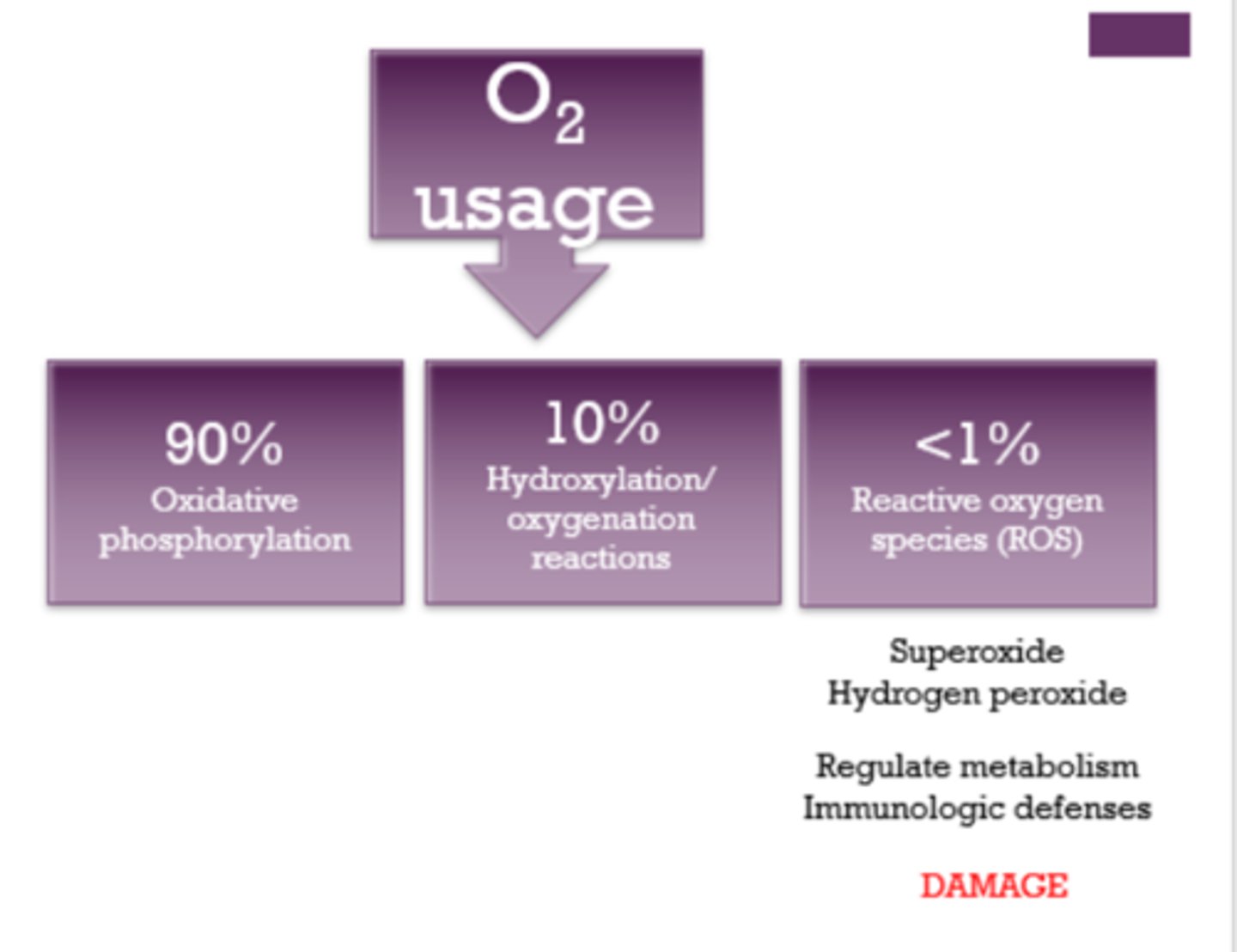
True or False: ROS does not cause damage.
False! ROS causes DAMAGE.
Oxidative stress:
1. It is caused by an imbalance between production and accumulation of ______ in cells and tissues.
2. The ability of a biological system to _____ these reactive products.
- ROS
- detoxify

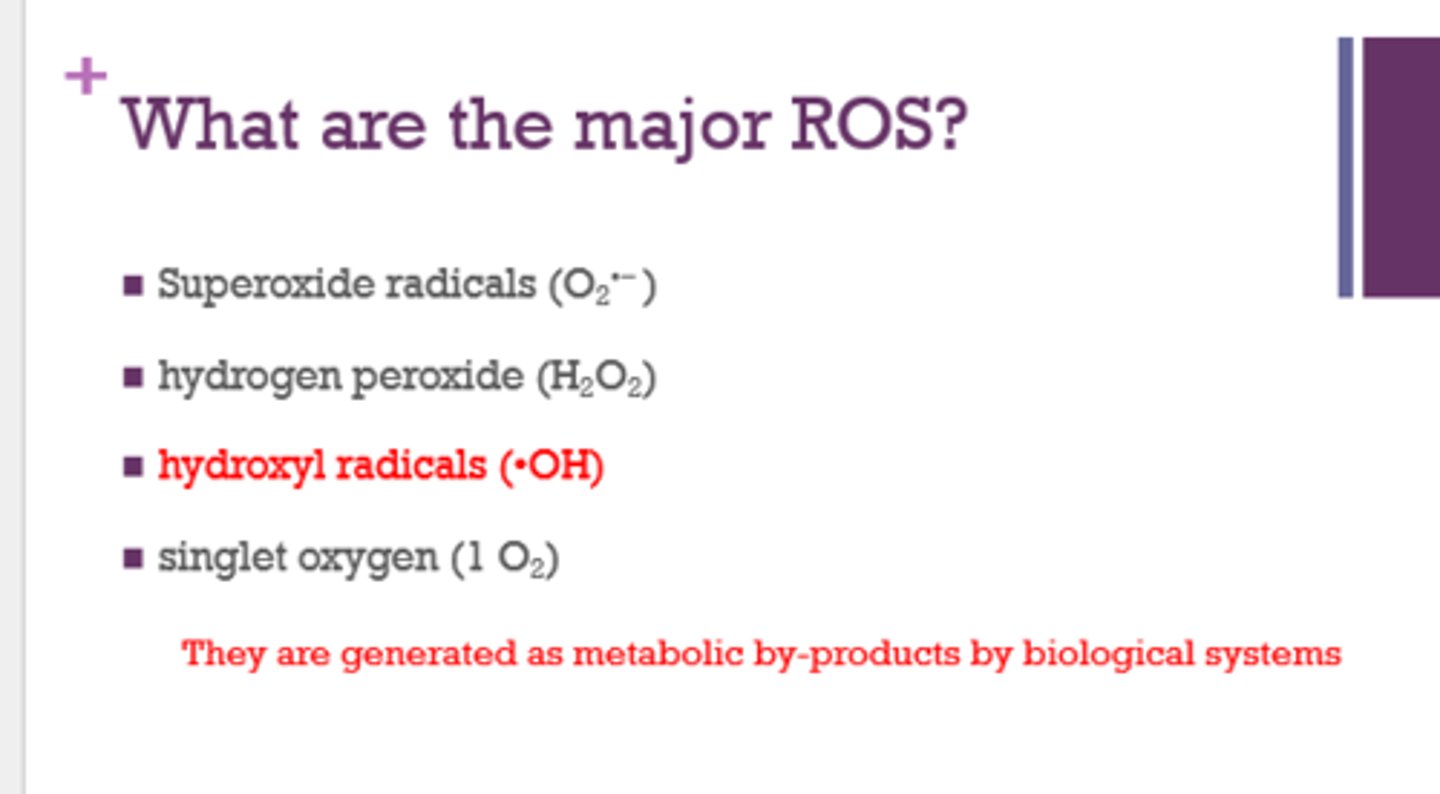
What are the 4 major reactive oxygen species (ROS)?
- superoxide radicals (O2-)
- hydrogen peroxide (H2O2)
- hydroxyl radicals (OH)
- singlet oxygen (1 O2)
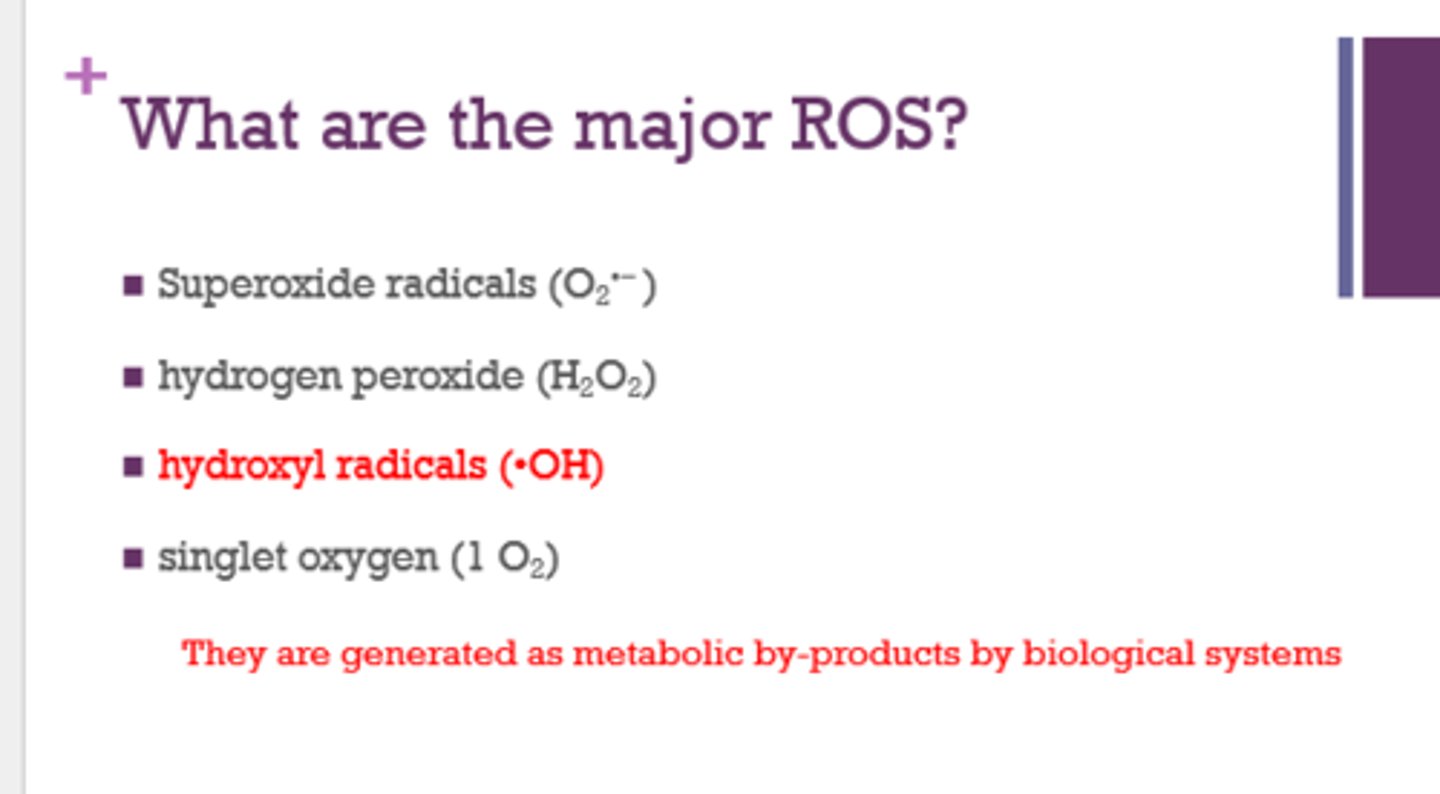
How are ROS formed in the cell?
as metabolic by-products by biological systems
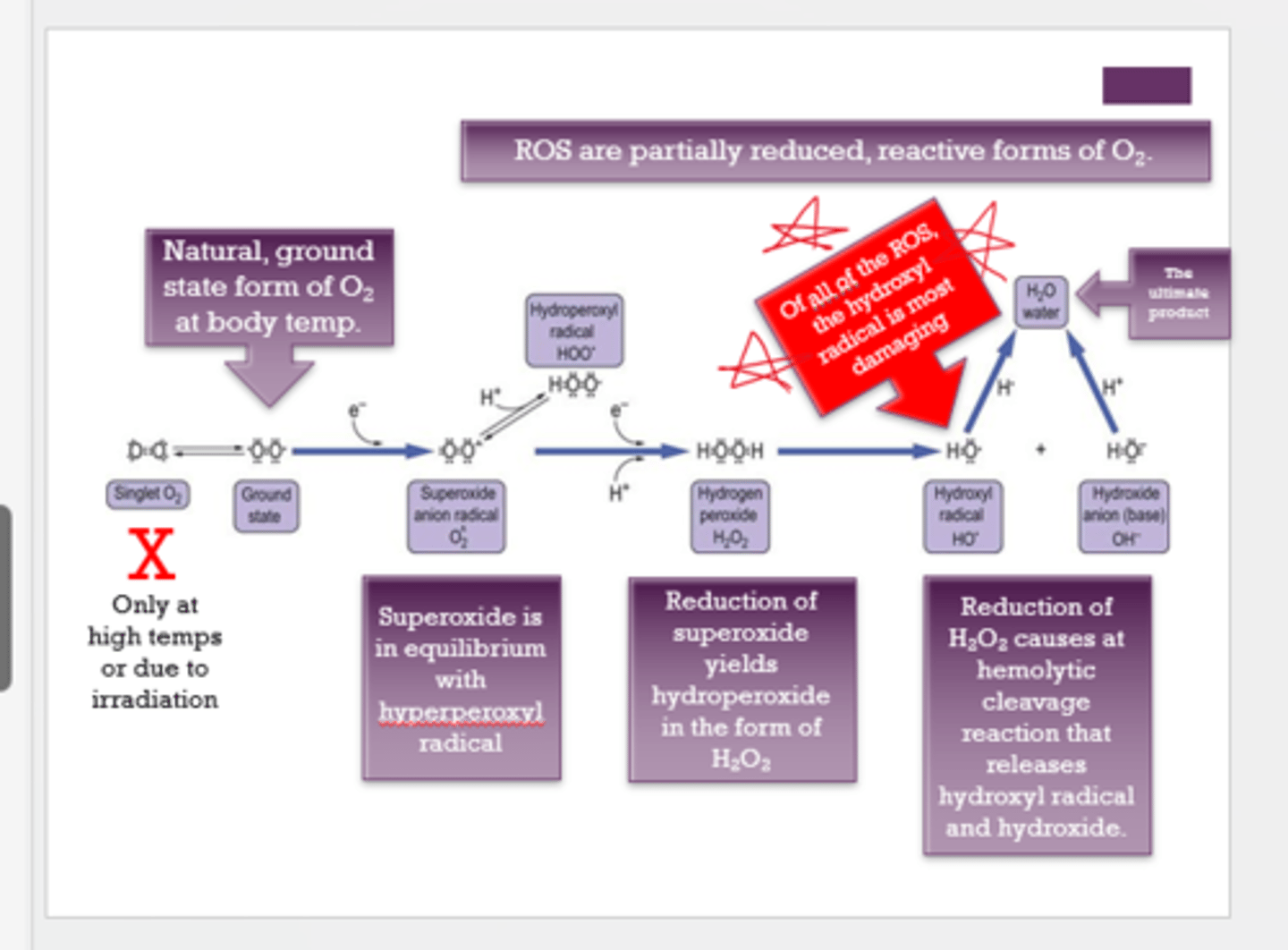
What is the most damaging/dangerous ROS?
hydroxyl radical
Where are ROS produced?
primarily in mitochondria

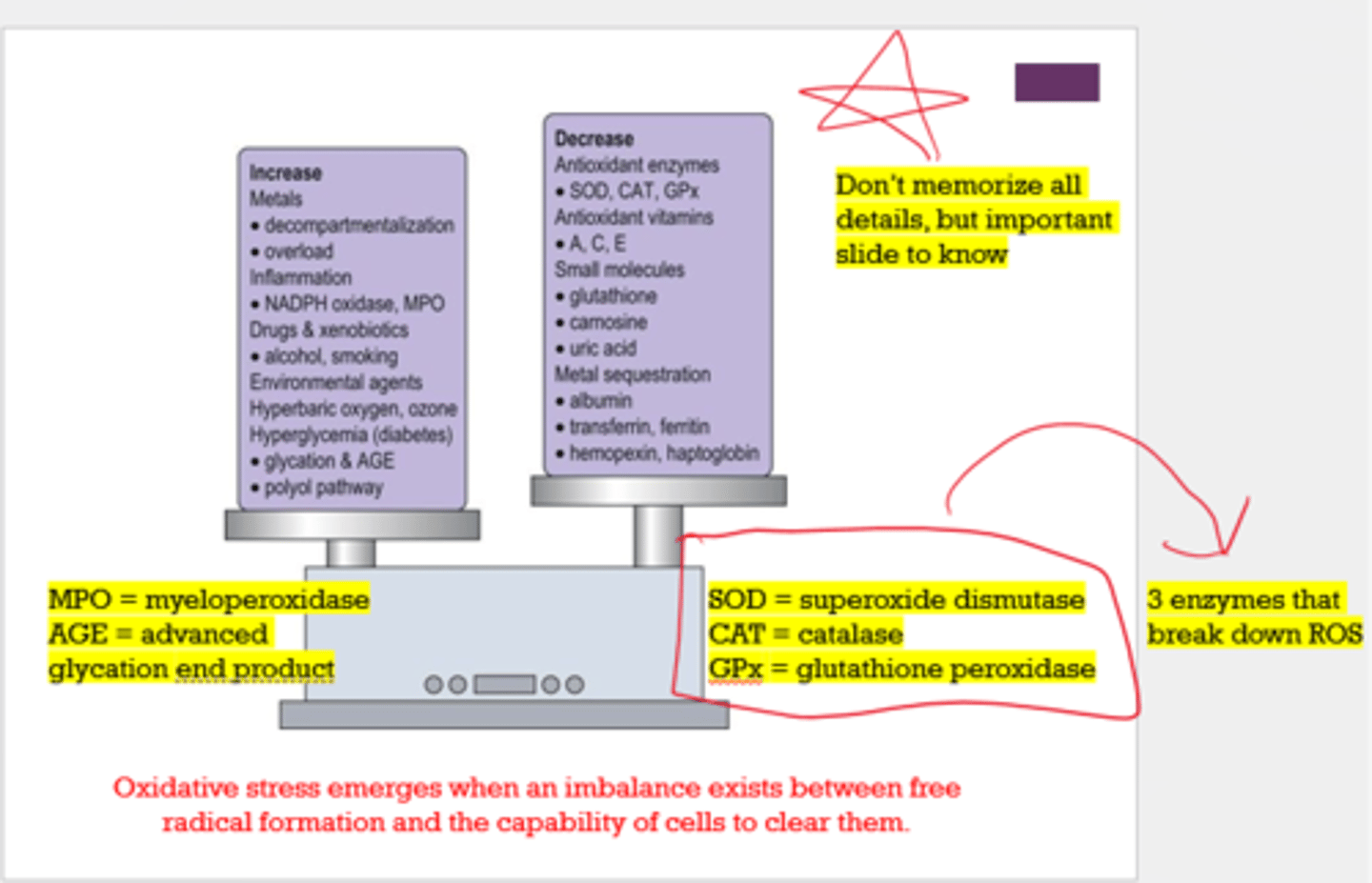
What are 3 enzymes that break down ROS?
- superoxide dismutase (SOD)
- catalase (CAT)
- glutathione peroxidase (GPx)
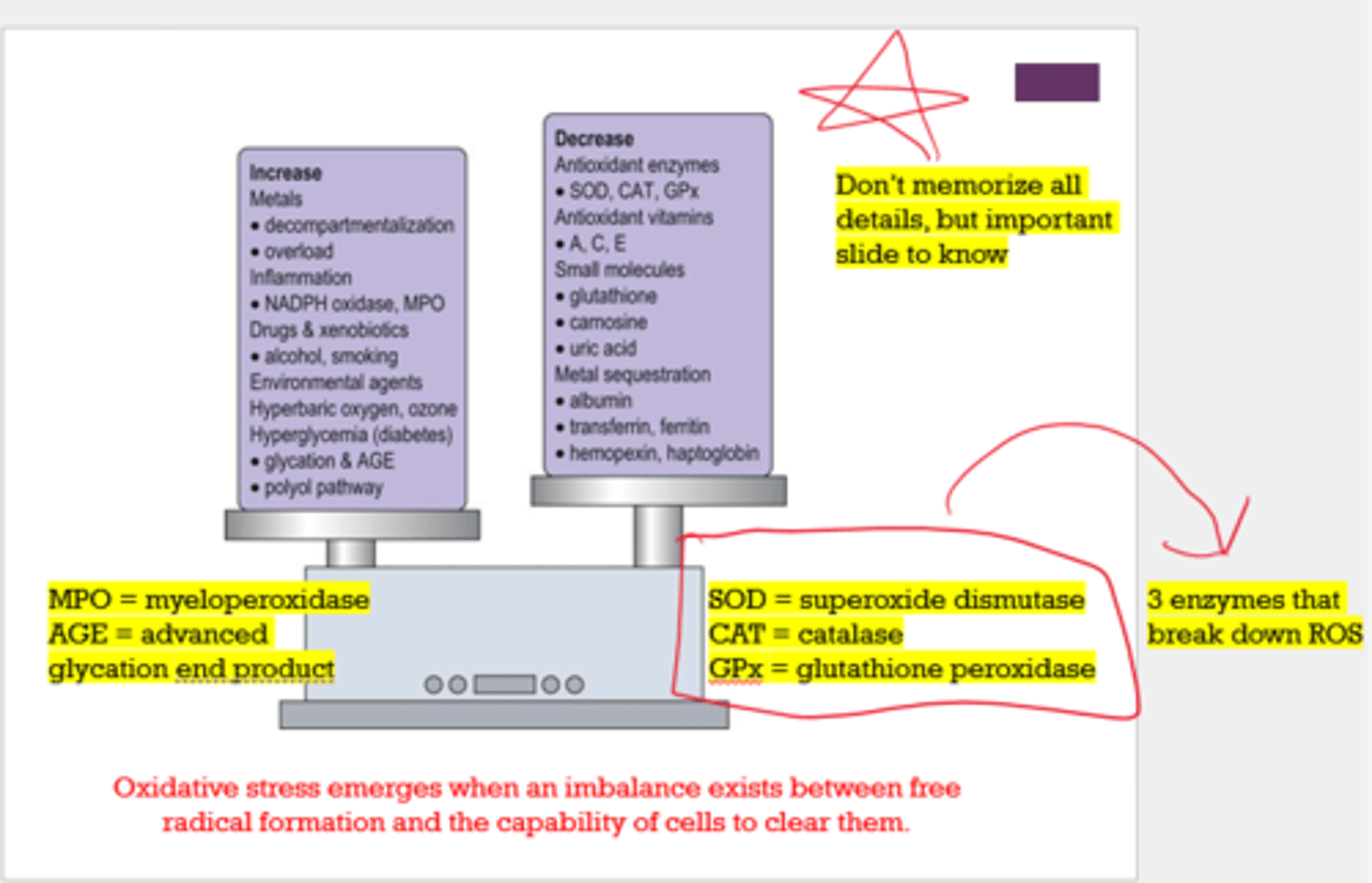
What 2 things increase oxidative stress?
- MPO (myeloperoxidase)
- AGE (advanced glycation end product)
What structures are particularly sensitive to free radical damage?
cell membranes that are rich in polyunsaturated fatty acids (PUFA)
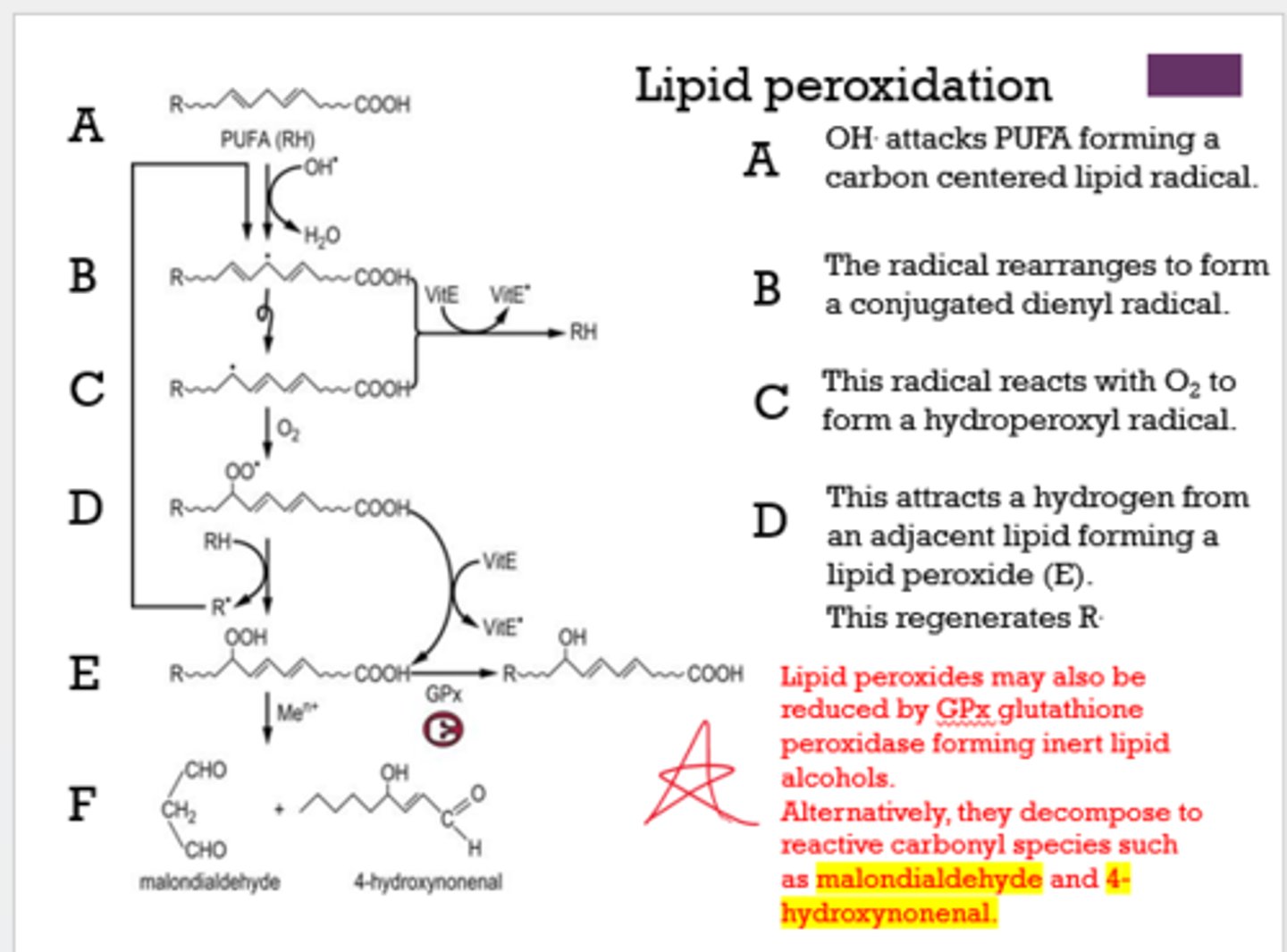
What can decompose to reactive carbonyl species, such as malondialdehyde and 4-hydroxynonenal?
lipid peroxides
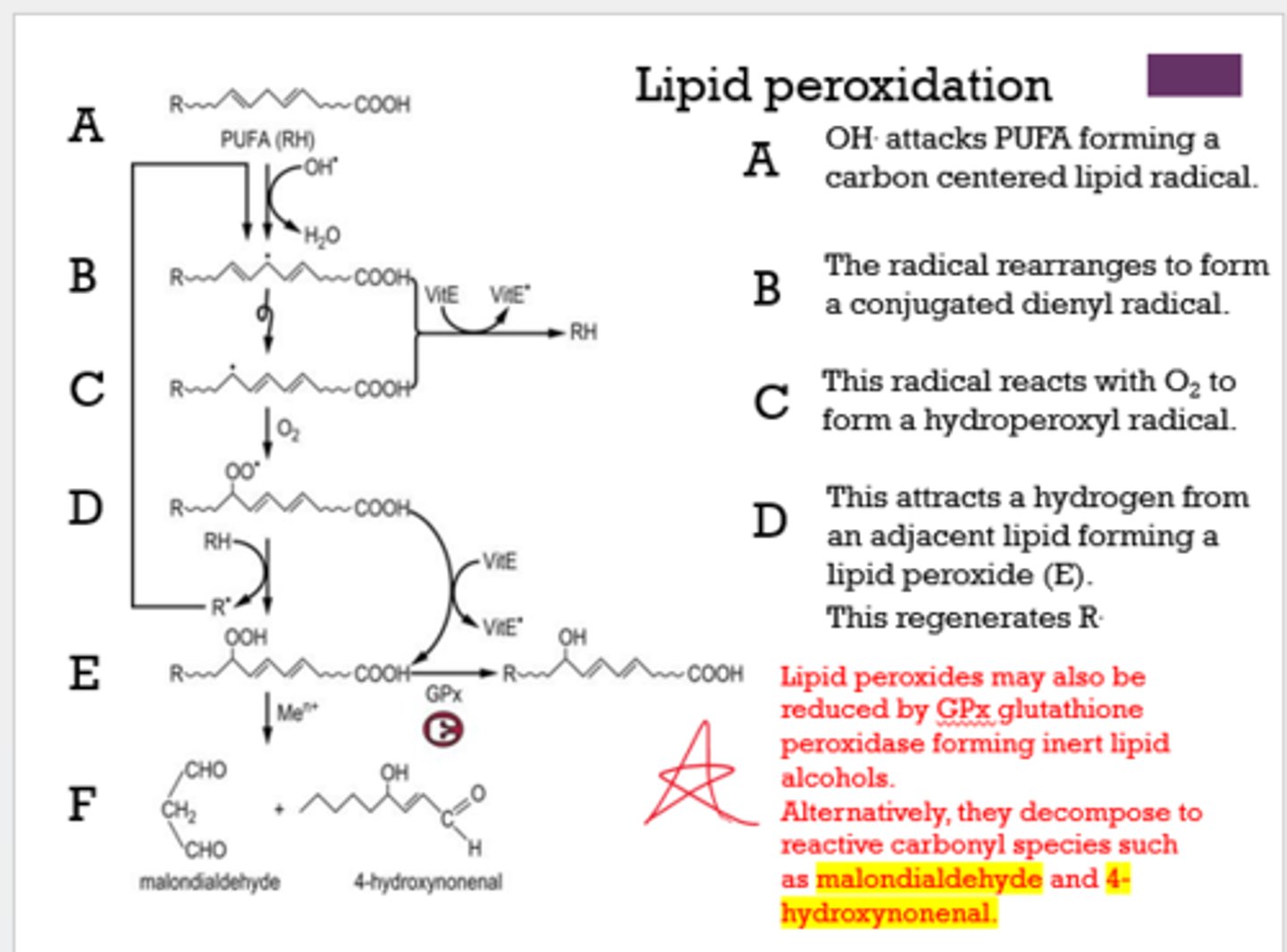
What happens to lipid peroxides when they are reduced by GPx?
they form inert lipid alcohols
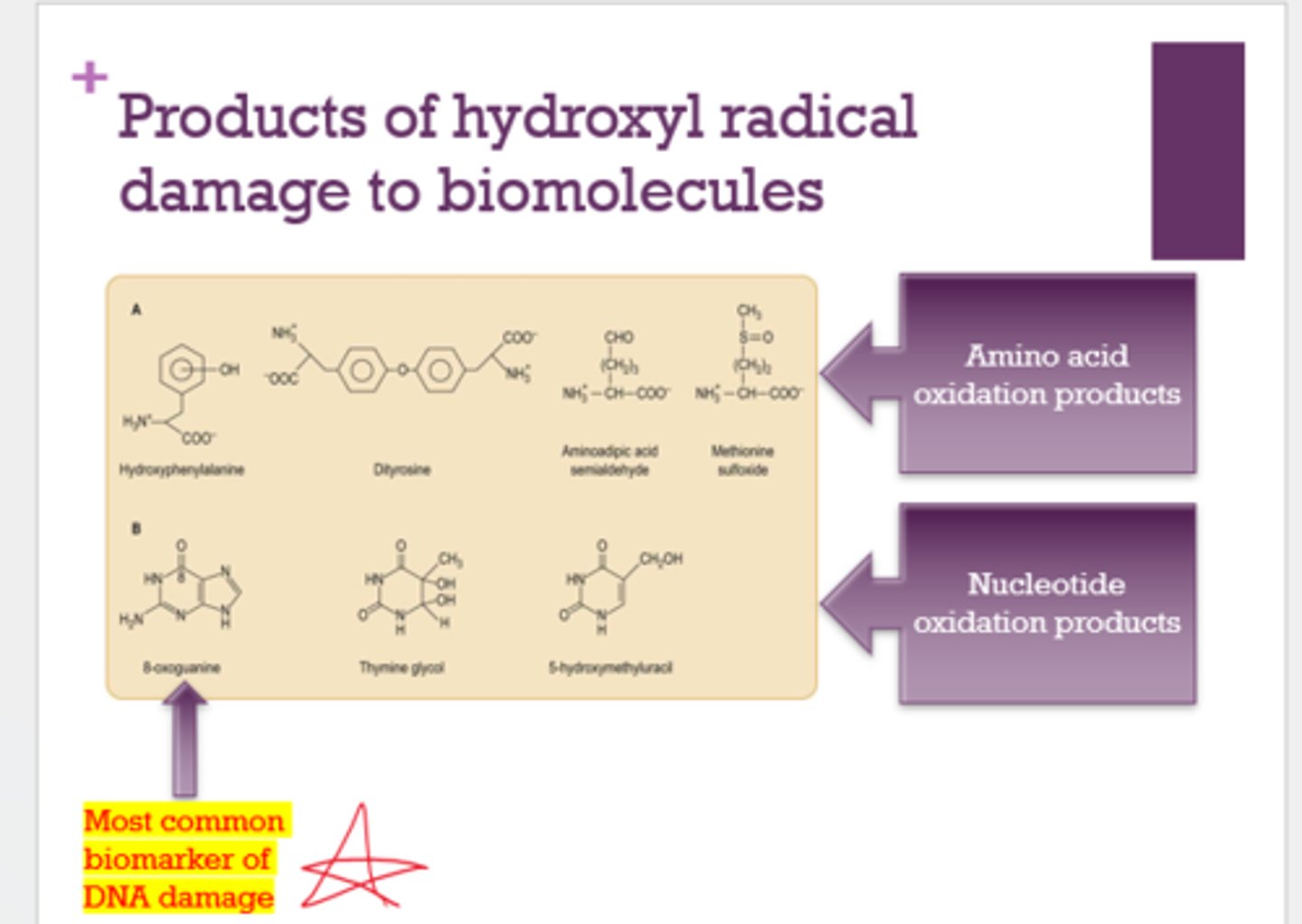
What is the most common biomarker of DNA damage?
(It's going to be a question like: "Out of these molecules, which one is most likely to...." - Dr. Breakwell)
8-oxoguanine
What is the first line of defense against oxidative damage?
sequestration or chelation of redox-active metal ions such as Fe and Cu

How is ROS damage to lipids and proteins repaired?
mainly by degradation and resynthesis

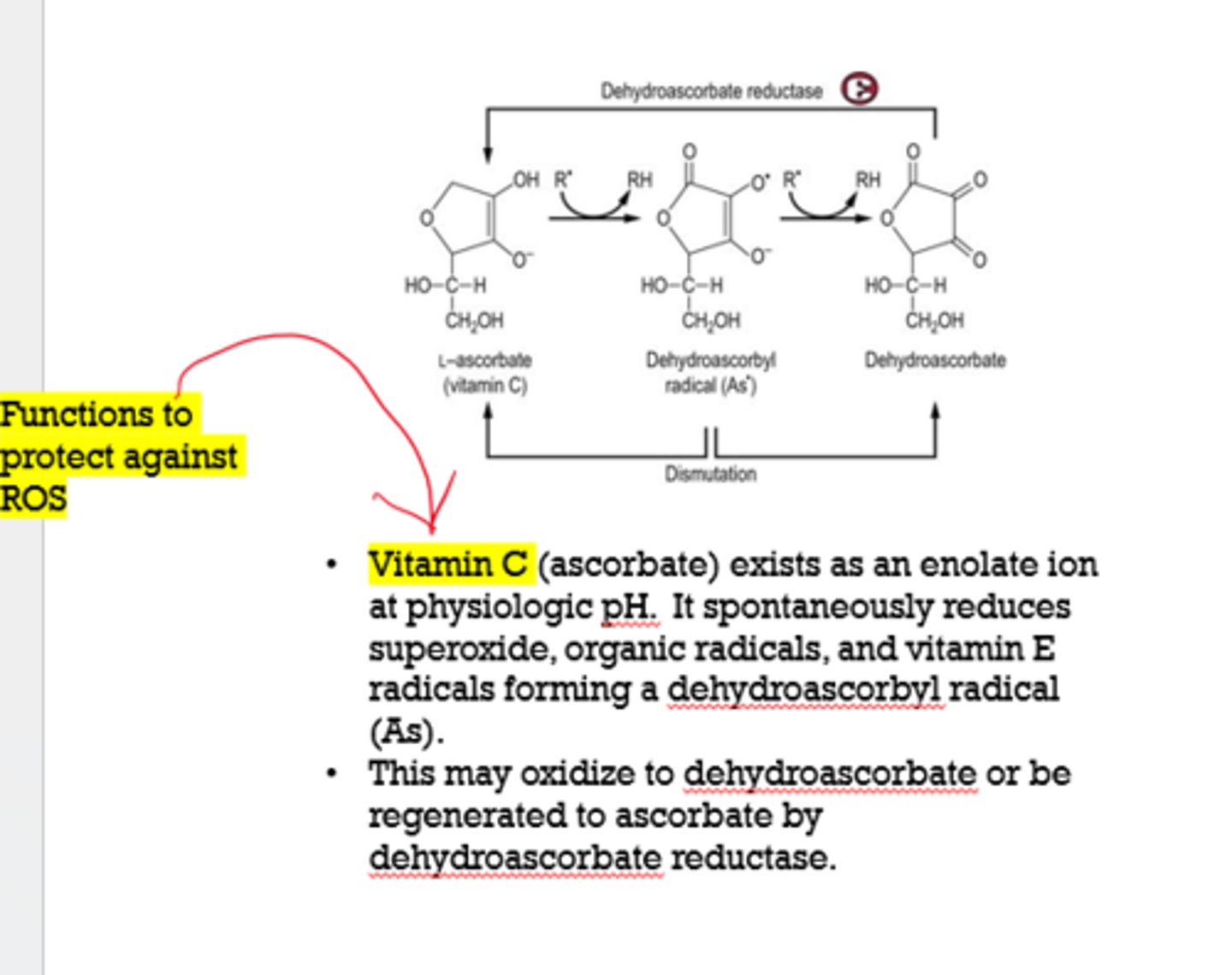
What vitamins function to protect against ROS?
- vitamin C (ascorbate)
- vitamin E (tocopherols)
Note: he didn't highlight anything on this slide for our class
What detrimental effects can free radicals have on human health? (7)
- cancer
- cardiovascular disease
- neurological disease
- respiratory disease
- rheumatoid arthritis
- kidney diseases
- delayed sexual maturation

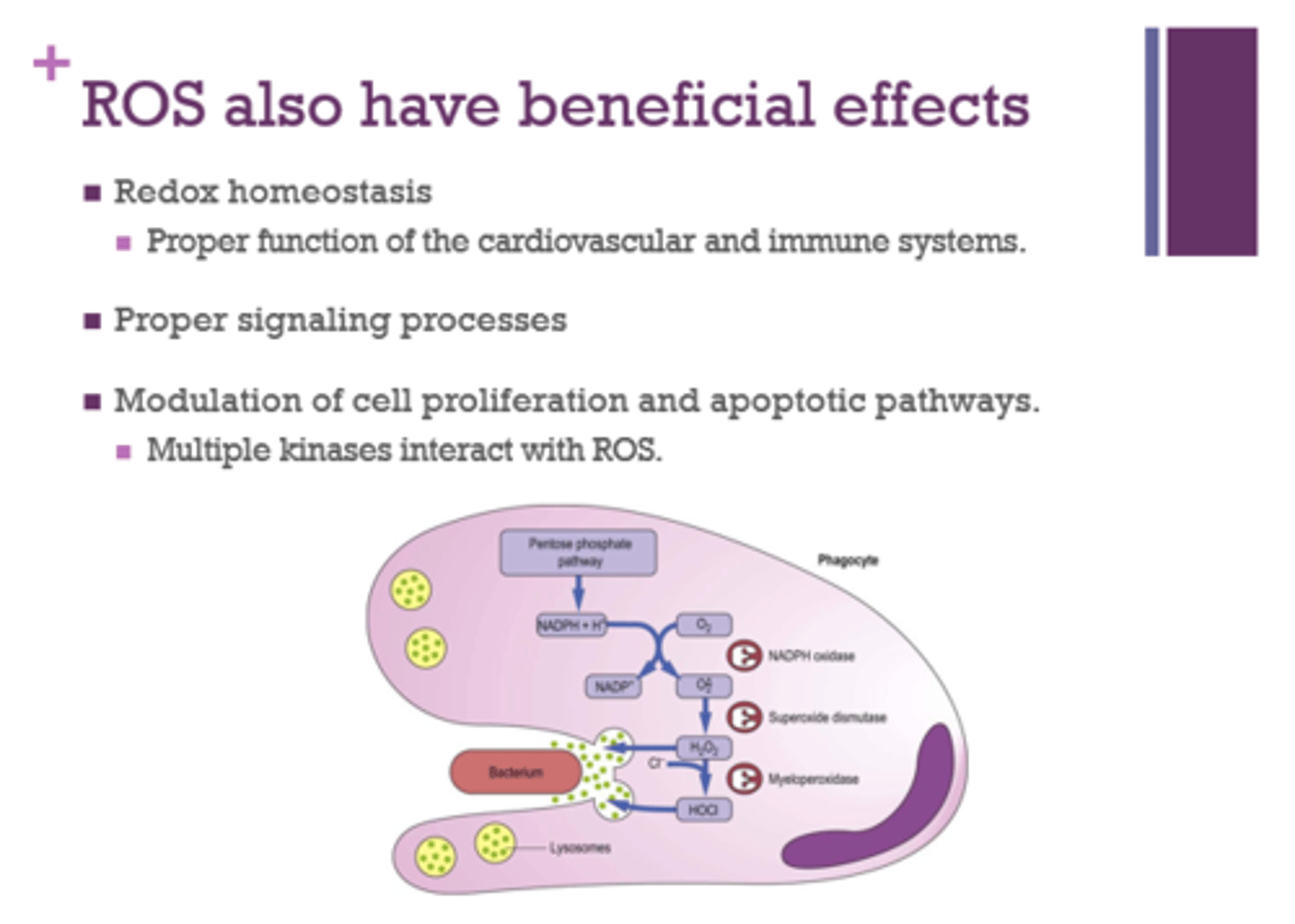
What benefits can ROS have?
- redox homeostasis
- proper signaling processes
- modulation of cell proliferation
- apoptotic pathways (programed cell death)