AQA A LEVEL Physics Wave Particle duality
1/35
Earn XP
Description and Tags
📚 1. Historical Development of Theories of Light Isaac Newton (1704) – Corpuscular Theory Proposed light is made of particles (corpuscles). Explained reflection and refraction. Could not explain diffraction or interference. Dominated thinking for over 100 years. Christiaan Huygens (1678) – Wave Theory Proposed light is a wave that travels through a medium (aether). Explained reflection, refraction, and polarisation. Rejected in favor of Newton’s theory at the time. 🔦 2. Thomas Young (1801) – Double-Slit Experiment Shone light through two slits → produced interference pattern. Proved that light must be a wave, as particles cannot interfere. Supported Huygens' wave theory. ⚡ 3. James Clerk Maxwell (1860s) – Electromagnetic Theory Developed equations showing light is an electromagnetic wave. Predicted that light is a transverse wave travelling at c = 3 × 10⁸ m/s. Unified electricity, magnetism, and optics. 📻 4. Heinrich Hertz (1887) – Discovery of Photoelectric Effect Demonstrated that UV light causes electrons to be emitted from metal. Called the photoelectric effect. Could not explain why intensity didn’t matter or why there was a threshold frequency. 🌈 5. Max Planck (1900) – Quantum Hypothesis Explained black-body radiation by assuming energy is quantised. Introduced idea that energy comes in discrete packets (quanta): E = hf Laid the foundation for quantum theory. ☀️ 6. Albert Einstein (1905) – Photon Model of Light Explained photoelectric effect using Planck's quantum idea. Proposed light consists of photons with energy: E = hf Electrons are emitted if hf > Φ (work function), with: K.E. = hf − Φ Won Nobel Prize in Physics (1921) for this work. Proved particle-like nature of light. 🌊 7. Louis de Broglie (1924) – Matter Waves Proposed wave-particle duality applies to matter too. Particles (e.g. electrons) have a wavelength: λ = h / p = h / (mv) Predicted electron diffraction would occur. 💡 Evidence: Electron diffraction experiments confirmed de Broglie’s hypothesis. When electrons passed through a crystal, they showed interference patterns like waves. 🧠 Final Conclusions Light behaves as both wave and particle → confirmed by: Wave effects: interference, diffraction. Particle effects: photoelectric effect. Matter also behaves like waves at small scales. Foundation of quantum physics, which replaced classical physics at atomic scales.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
Outline Newton’s Corpuscular theory?
This theory pustulates that light is made of small particles (corpuscles).
He proposed that white light is made up of different colours, which can be separated by a prism.
Outline Huygens wave theory on light
This theory postulates light behaves as a wave.
All points on a wavefront produce their own secondary waves (Wavelets).
Light was a longitudinal wave but as longitudinal waves require a medium to travel through (space was ‘empty’), Huygens suggested space was filled with an invisible massless substance, aether this allowed the light to travel through space.
Explain reflection based on Newton’s corpuscular theory.
Newton suggested that corpuscles behave as balls that bounce off surfaces.
This occurred because the corpuscles carried momentum and so would change direction upon hitting a reflective surface, obeying the conservation of momentum.
The speed only changes in the direction perpendicular to the surface, it must be reflected at the same angle.
Describe the differences between Newton’s and Huygens theories on refraction
Newton believed light was made of particles (corpuscles), while Huygens proposed it was a wave.
Newton’s theory predicted that light travels faster in denser media, as particles would be attracted more strongly.Huygens’ wave theory suggested light slows down in denser media, as waves are slowed by the medium.
Newton explained refraction by a change in the perpendicular component of velocity, while the parallel component stayed the same.Huygens explained refraction using wavefronts, where part of the wave slows at the boundary causing the wave to bend towards the normal.
Experiments later showed light slows in denser media, supporting Huygens’ theory.
How did Young’s experiment support Hyugen’s wave theory.
The double slit experiment displayed a pattern of fringes due to interference between different wavefronts.
Young’s experiment occurs due to diffraction which only waves can do.
Therefore Huygen was correct in his wave theory.
How are fringes formed in Young’s double slit experiment?
Monochromatic light passes through the two slits.
The two slits act as coherent light sources,
this causes bright fringes (Maxima) at points where light is in phase and constructive interference occurs, and dark fringes (Minima) where destructive interference occurs where light is out of phase.
Why does Newton’s Theory fail to explain Young’s interference pattern?
If Newtons corpuscular theory was correct then there would only be fringes at the points where light passes through the fringe spacing but instead a pattern of repeating light and dark fringes is observed. Newton’s theory was inaccurate.
What lead to Newton’s theory being rejected?
His theory was rejected due to the inability to explain interference and diffraction patterns observed in experiments like Young's double slit experiment.
Also, after Young’s discovery, light speed was measured in water and found to be less than air.
What did Maxwell predict about electromagnetic waves?
Maxwell predicted that electromagnetic waves travel at the speed of light and consist of oscillating electric and magnetic fields perpendicular to each other and the direction of propagation.
He also derived the equation for the speed of an EM wave and determined that light was an EM wave along with UV, Infrared and others.
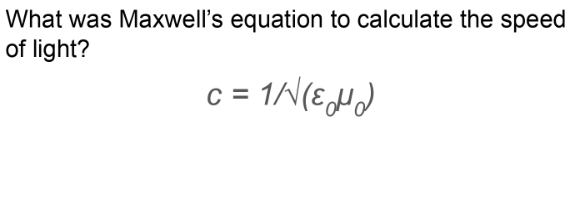
What are the permittivity and permeability of free space?
ε₀ (permittivity)-represents the strength of an electric field due to a charged object in free space.
μ₀ (permeability)-represents the magnetic flux density due to a current carrying wire in free space.
How are stationary waves produced?
When a wave is reflected against a transmitter two coherent progressive waves travelling in opposite directions interfere.
This causes points of maximum displacement (antinodes) and points of minimum displacement (nodes).
How did Hertz discover radio waves?
Hertz discovered radio waves by using a spark gap transmitter, where a spark created high-frequency oscillations that radiated energy (radio waves)
he detected these waves using a dipole detector.
As the radio waves produced a voltage across the dipole, it caused a spark in the detector.
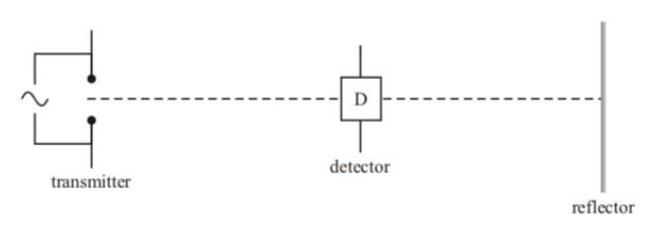
Explain why the strength/reading of the detector varies repeatedly between a minimum and maximum as it travels along the line.
As the transmitter emits a wave it is reflected of the reflector,
producing 2 coherent waves acting in opposite directions that interfere.
This interference causes points of maximum displacement (antinodes) and minimum displacement (nodes).
As the detector moves along the line its reading increases the closer it gets to an antinode and decreases as it moves closer to a node.
What did Hertz discover about radio waves after further experiments?
They can be reflected by metal,
produce stationary waves,
Cannot be stopped by insulators, (So do not have charge)
Can be polarised.
From this he concluded they were EM waves.
How did Hertz determine the speed of radio waves?
Hertz measured the distance between adjacent nodes (1/2 wavelength).
He then multiplied by the frequency of the waves to determine their speed.
This was similar to Maxwell’s predicted value from his equation.
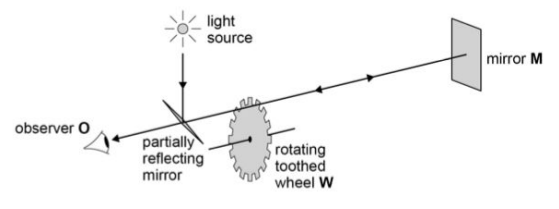
This is the apparatus that Fizeau used to determine the speed of light.
(1) State what he observed when the speed of rotation is low.
(2) This continued until a certain speed was reached.
,State his observation when the rotation speed reached that certain speed.
(1) When the rotation speed was low, the light was able to return through the original gap.
(2)The light is blocked by the cog so is unable to pass through the gap, indicating the speed of light.
Define a Black body and state it’s relevance in the ultraviolet catastrophe.
A Black body is an idealized physical object that absorbs and emits all incident electromagnetic radiation, regardless of frequency or angle of incidence. Wavelength emits depends on its temperature.
It was hypothesised that a perfect black body at lower temperatures huge amounts of low wavelength radiation would be produced, leading to the prediction of infinite energy emission at ultraviolet (very low) wavelengths, which contradicted experimental observations.
How did Planck resolve the UV catastrophe?
He suggested that EM radiation is released in quanta of energy called photons proportional to it’s frequency and not continuous therefore it is not infinite.
When was the photoelectric effect first discovered?
Hertz observed that the strength of the sparks varied with different types of EM waves on the detector, however the strength remained constant if the same EM wave remained incident on the detector.
If frequency is below the threshold, would photoelectrons still be emitted with enough intensity?
Electrons are emitted by 1-1 interactions with photons when they carry a sufficient amount of energy which is proportional to their frequency. Therefore regardless of the intensity photons will not be emitted.
What does maximum kinetic energy of an electron depend on?
It depends on the frequency of light used because f is directly proportional to E.
How does increasing intensity of light affect the rate of photoelectron emissions?
A higher intensity means more 1-to-1 interactions with the electrons, therefore more photoelectrons are emitted, increasing the rate of emission.
Why does the photoelectric effect oppose wave theory?
The effect causes instantaneous release of electron given that the frequency is above a certain threshold.
Wave theory suggests emission won’t always happen instantaneously and predicts that any frequency of light will eventually cause electrons to be emitted.
This is against the experimental results of Hertz and so opposes the wave theory.
What is stopping potential and how is it measured?
Stopping potential is the minimum voltage needed to stop the flow of photoelectrons emitted from a metal surface in the photoelectric effect.
It is measured by applying an electric potential difference that balances the kinetic energy of the emitted electrons.
State De Broglie’s hypothesis
De Broglie's hypothesis states that particles, such as electrons, exhibit wave-like properties, and their wavelength is inversely proportional to their momentum.
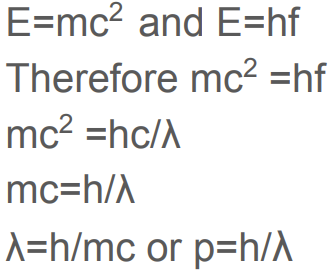
What was observed by the electron diffraction experiment and why was this observed? State The significance of this experiment.
Electrons produced a pattern of concentric rings, due to the constructive and destructive interference that occurred when the electron beam is diffracted.
Significance: Only waves were known to diffract. This supported de Broglie’s hypothesis that particles could also behave like waves.
How is the cathode ray in an electron diffraction experiment created?
A filament is supplied with energy from a power supply, increasing the current and the temperature of the filament.
This supplies the filament with enough kinetic energy to emit electrons from the surface of the metal.
These electrons are accelerated from cathode towards the anode using a p.d.
When the electrons have fully accelerated they will all be travelling with the same kinetic energy which is equal to the work done by the p.d.

What is de Broglie’s Wavelength in reference to anode potential?
.
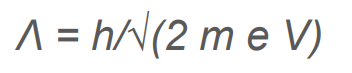
How does increasing the electron’s speed change the diffraction pattern?
Increasing the speed of the electron, decreases it’s wavelength.
This will produce rings that are closer together.
What anode voltage is required for electrons to have wavelengths of the same order as a atom?
150V
Define Resolving power.
The ability of an optical system to distinguish between closely spaced objects, often defined as the minimum angular separation that can be resolved.
How are images formed by a TEM (Transmission Electron Microscope)?
An electron beam produced by thermionic emission is accelerated using a anode.
A Magnetic lens focuses the electrons, any electrons falling at angles towards the edge are deflected towards the centre. Electrons in the centre continue in their straight path.
A condenser lens focuses beam on the sample and the beam passes through the sample.
There is than an objective lens that forms an image of the sample.
A projector lens creates the final image on a fluorescent screen.
State the factors that lead to a lower resolution image
Lower anode potential- This would mean the electrons gain less kinetic energy, resulting in longer wavelengths and reduced resolving power.
Thickness of a sample- The electrons pass through thick sample, so have less kinetic energy as they slow down, decreasing their wavelength and reducing detail.
lens aberrations- These reduce the focusing ability of the lens to direct electrons travelling at different speeds due to being scattered.
Why is the pressure in the TEM low?
To reduce the amount of collisions with air particles.
If pressure is high rate of collisions increases, decreasing the kinetic energy of the electron, increasing their wavelength and reducing the resolving power.
How does a STM (Scanning Tunneling Microscope) work?
Uses Quantum Tunneling-a phenomenon where electrons tunnel through a barrier. The closer the barrier to source of emission the higher the probability of tunneling.
A fine probe is placed close to the surface of a material to produce a tunneling current between the surface and the probe.
A small p.d. goes between the probe and the surface so electrons cross the gap from negative to positive.