201K: To Alkenes, Arenes and Beyond - Aromaticity
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Arene
Aromatic hydrocarbon (C, H atoms only)
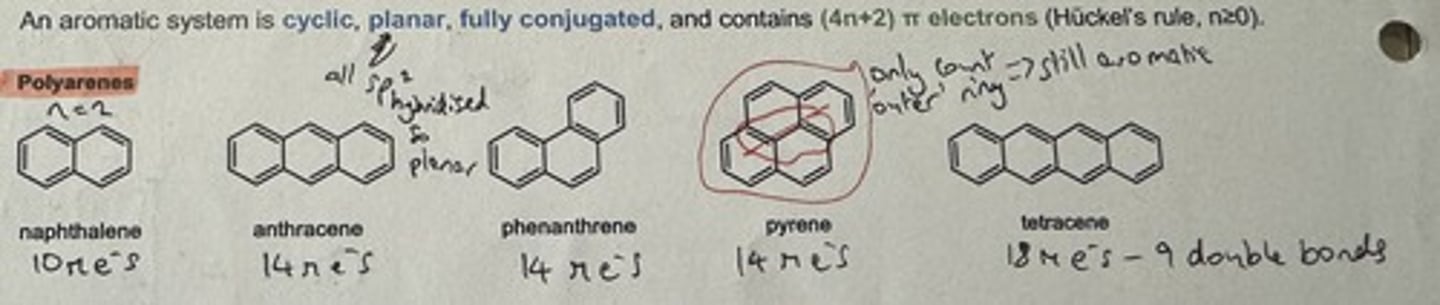
Polyarene
Polycyclic aromatic hydrocarbon
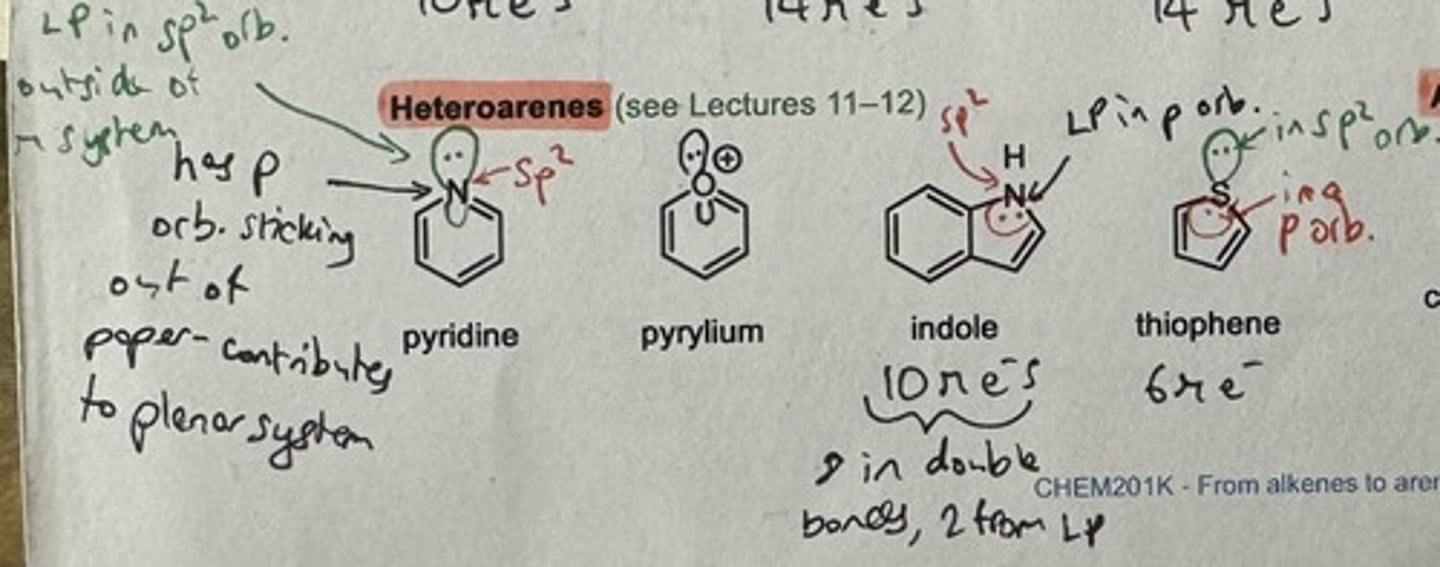
Heteroarene
Aromatic compound that also contains a heteroatom (N, O, S...)
Names of positions on aromatic ring (starting from atom connected to R group)
ipso
ortho, o
meta, m
para, p
Do arenes undergo addition reactions?
No. (unlike alkenes and conjugated polyenes)
→ undergo substitution rxns w/ E⁺ instead
Which data shows that benzene is more stable than might be expected for a hypothetical "cyclohexatriene"?
Heat of hydrogenation data
→ Hydrogenation of benzene is less exothermic than predicted
- aromaticity confers a special stabilisation to a molecule
Define an aromatic system
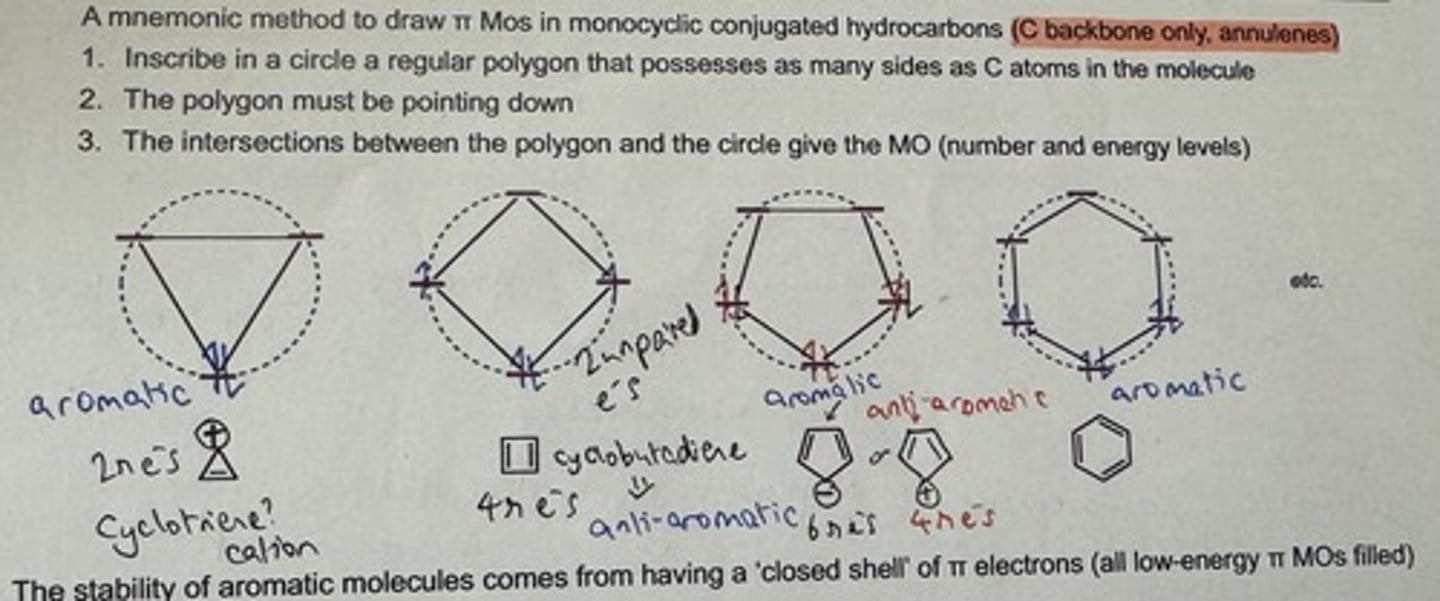
An aromatic system is cyclic, planar (sp²), fully conjugated and contains (4n+2) π electrons (Hückel's rule, n≥0)
(4n+2) π electrons: 2, 6, 10, 14, 18...
i.e. an ODD NUMBER of pair of e-s (1 pair, 3 pairs, etc.)
Why does an aromatic system have a special stability (aromaticity)?
- Has a 'closed shell' structure
→ i.e. having all low-E π MOs filled - requires exactly (4n+2) π electrons
Show some aromatic polyarene examples
Show some aromatic heteroarene examples
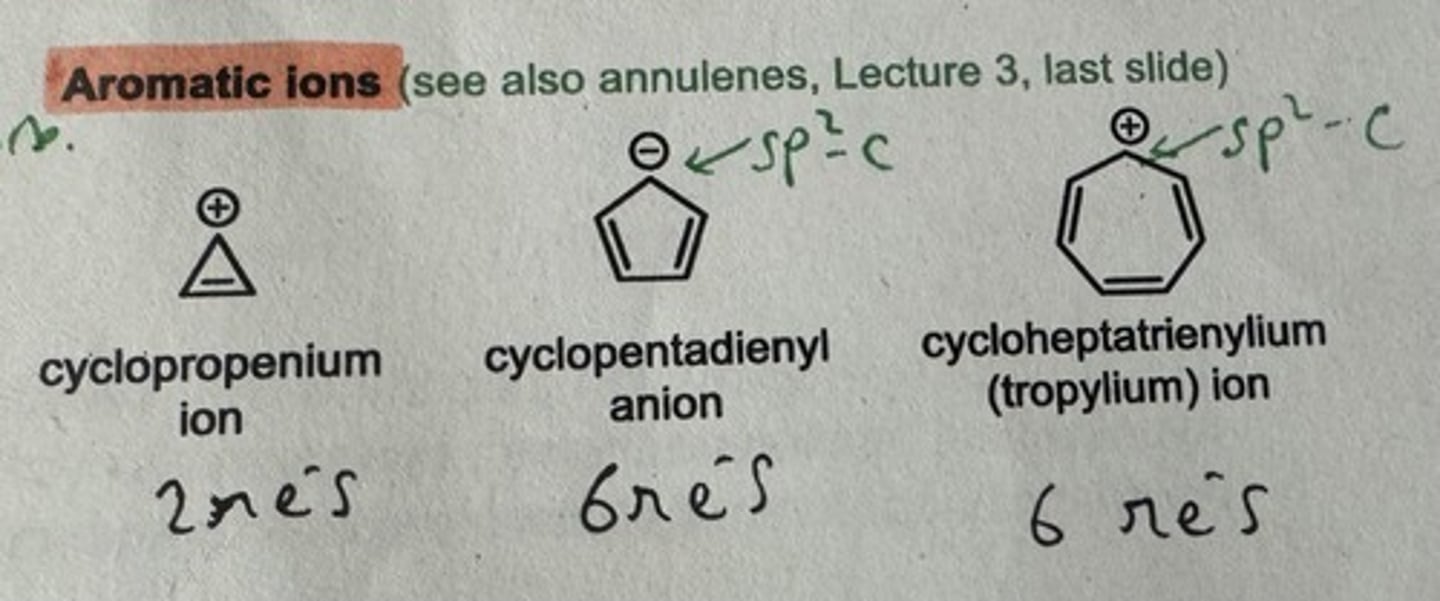
Show some aromatic ion examples
Why is pyridine aromatic?
N atom has a p orbital which contributes to the planar system
(However, the LP in the sp² orb is outside the pi system)
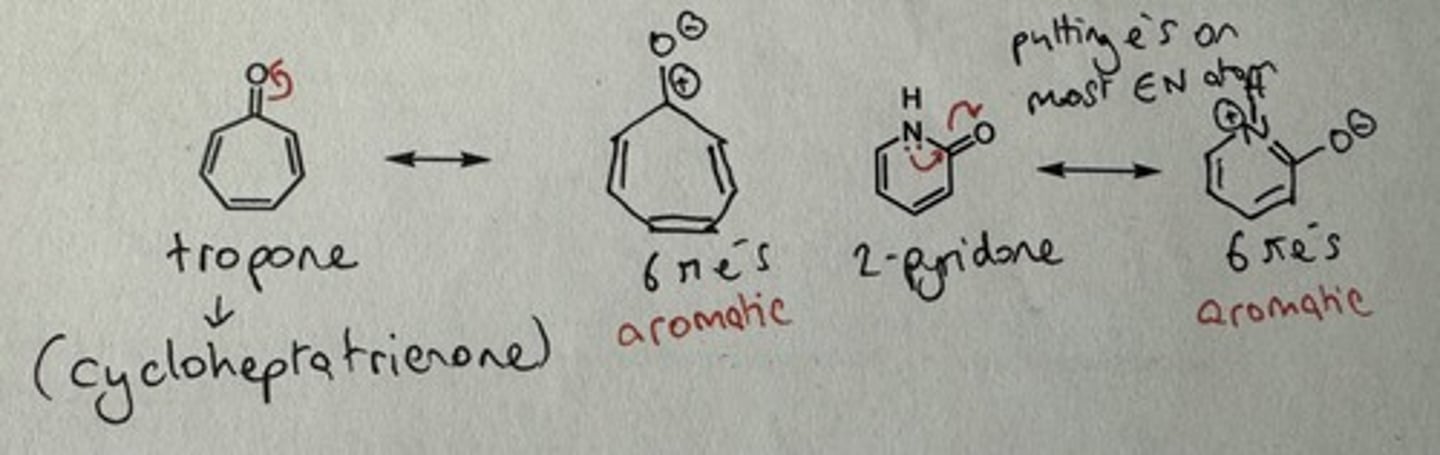
Show 2 examples of compounds that are aromatic due to a resonance form
Tropone and 2-pyridone
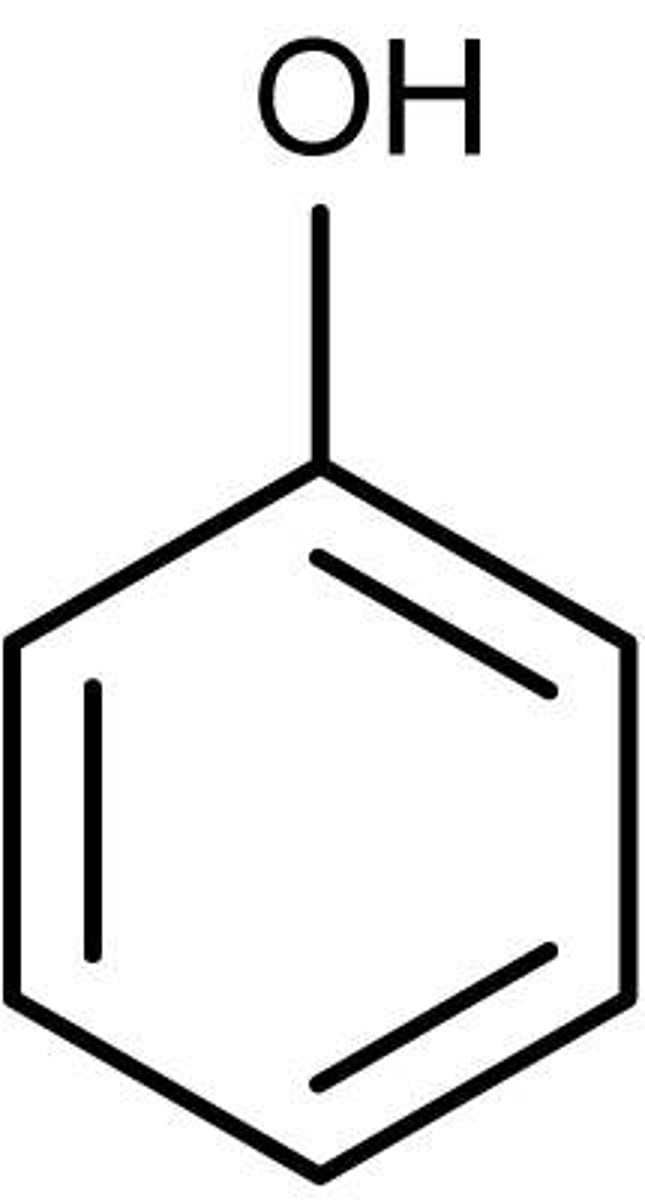
phenol
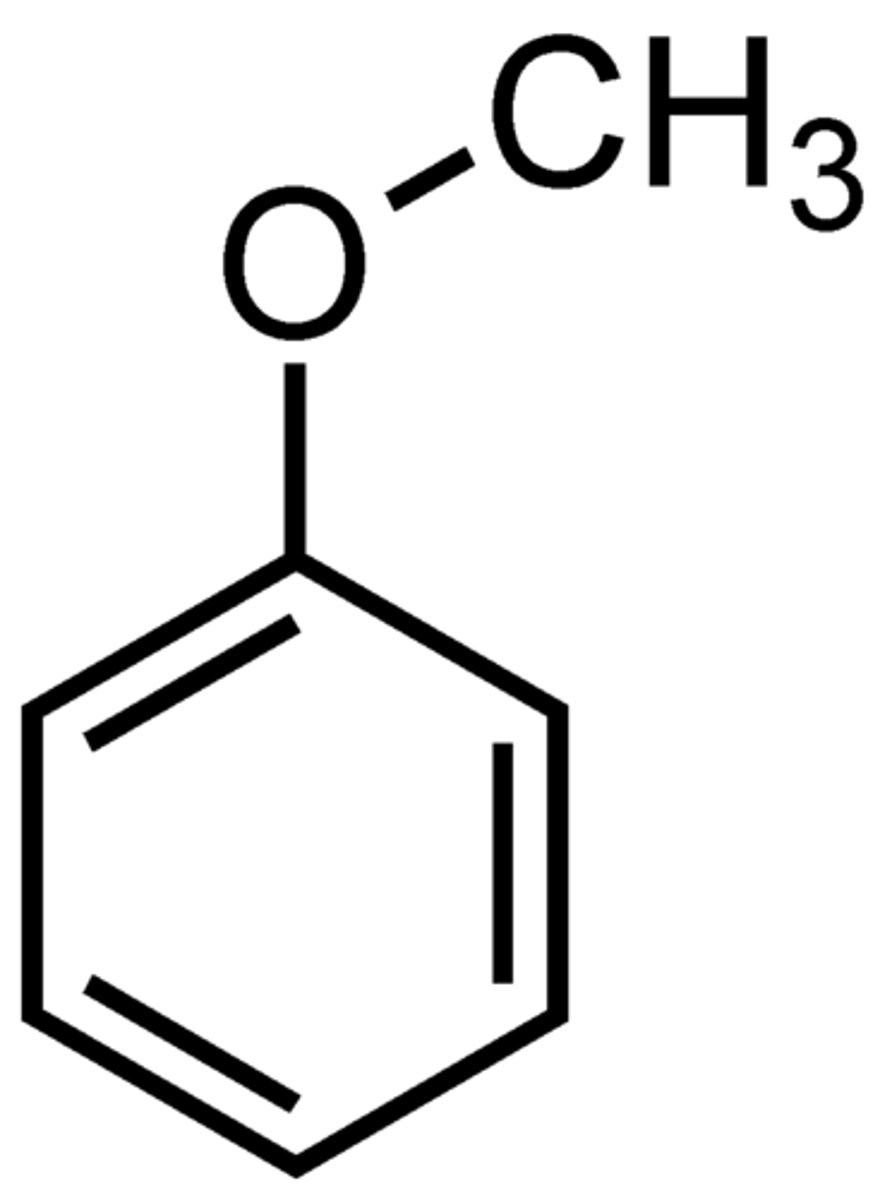
anisole
Ph-OMe
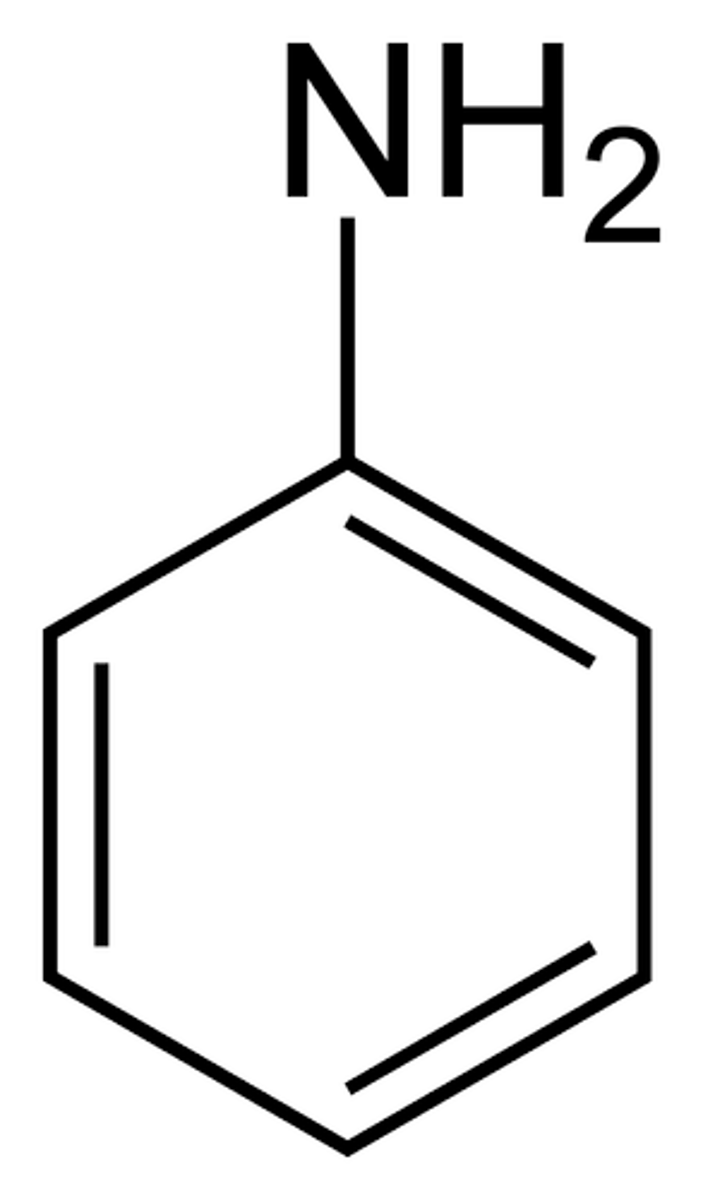
aniline
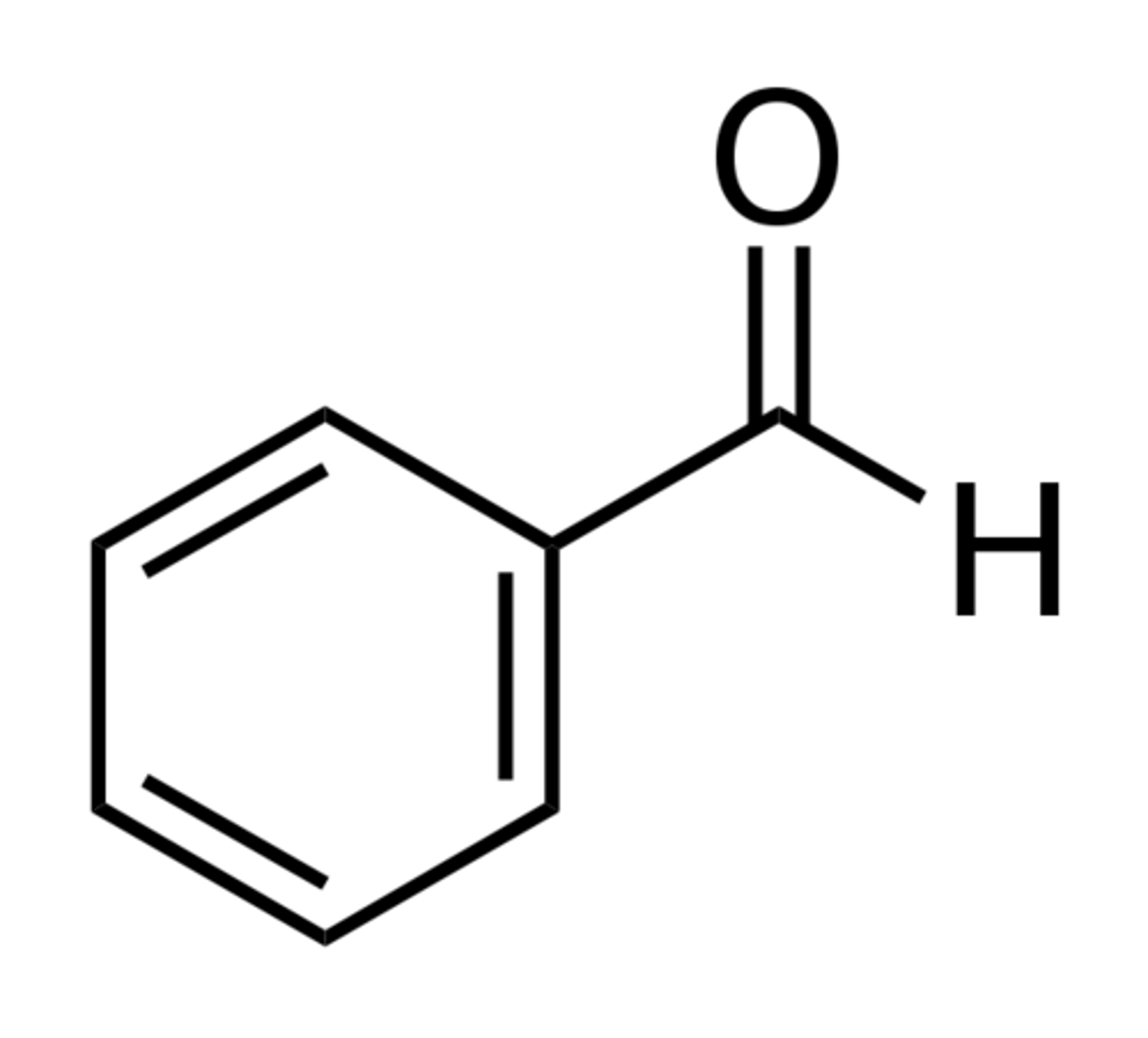
benzaldehyde
acetophenone
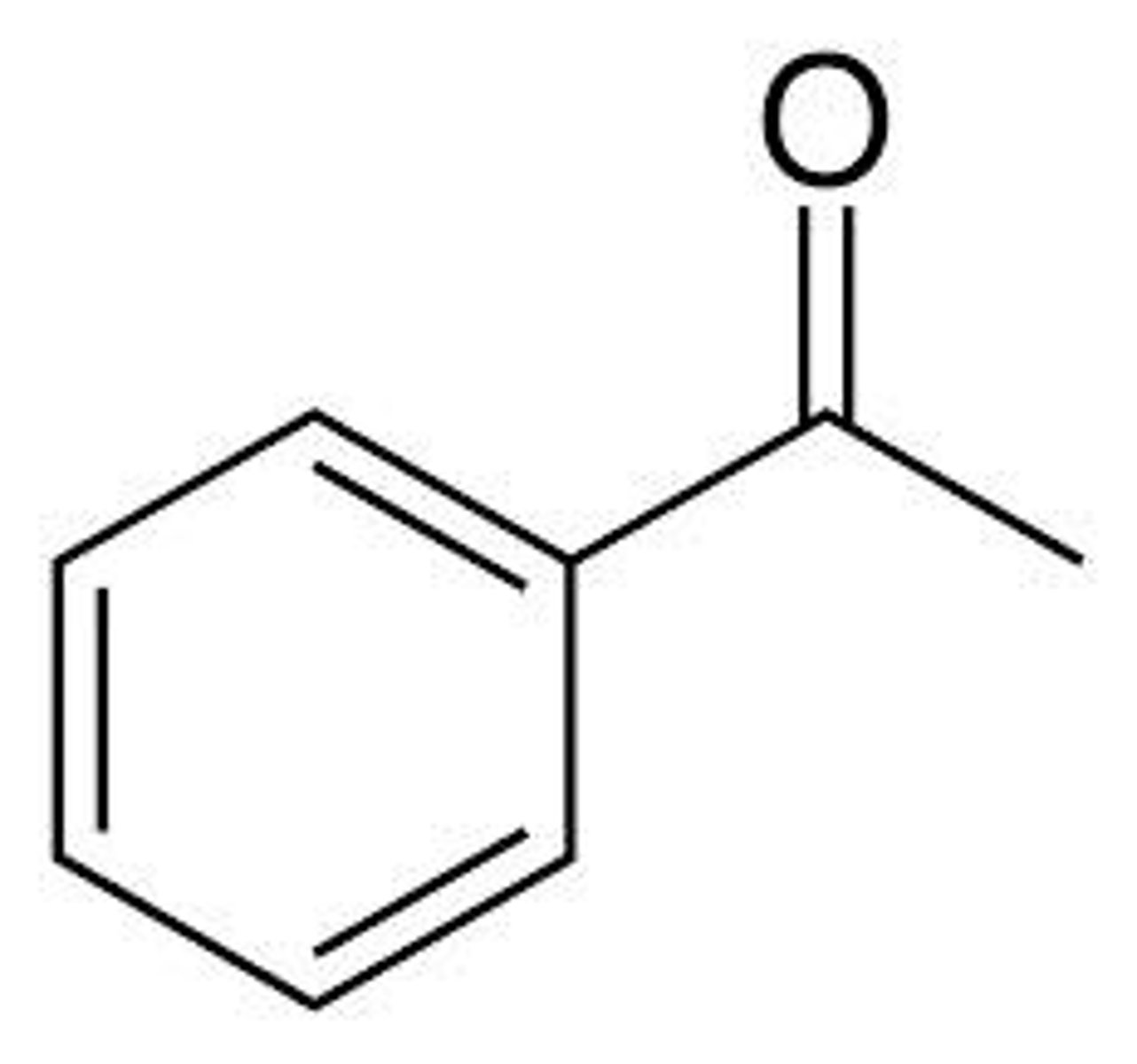
benzoic acid
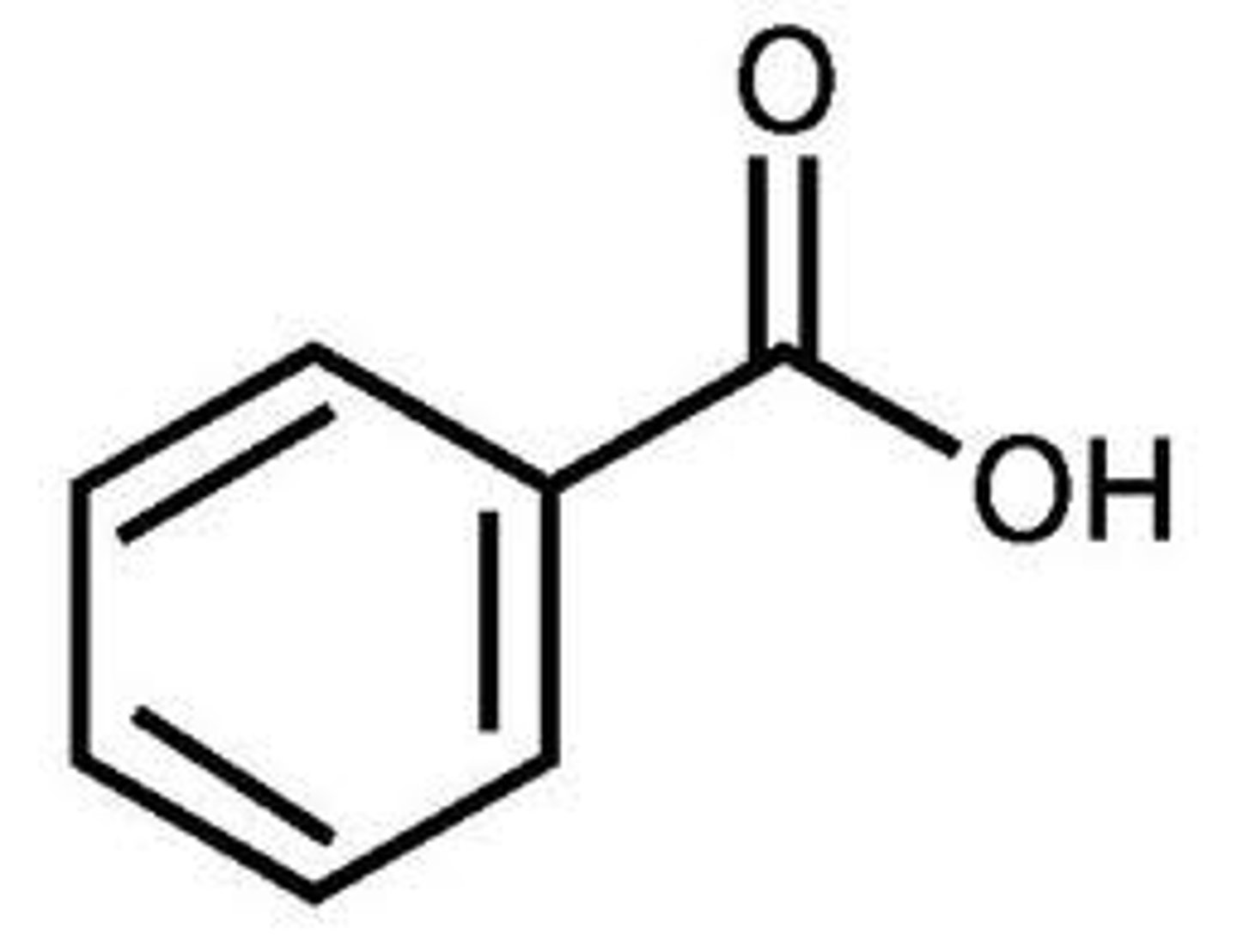
benzonitrile
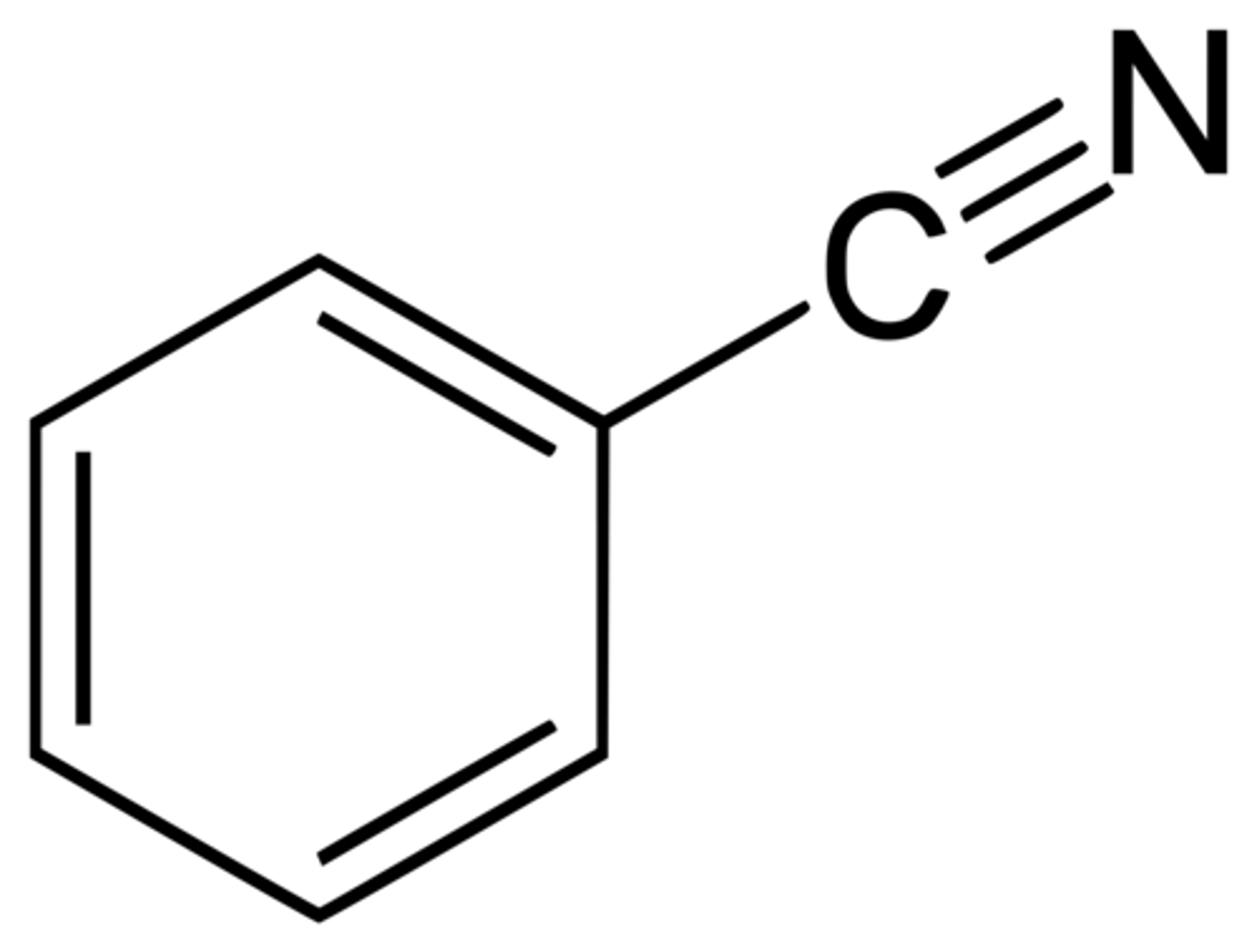
benzamide
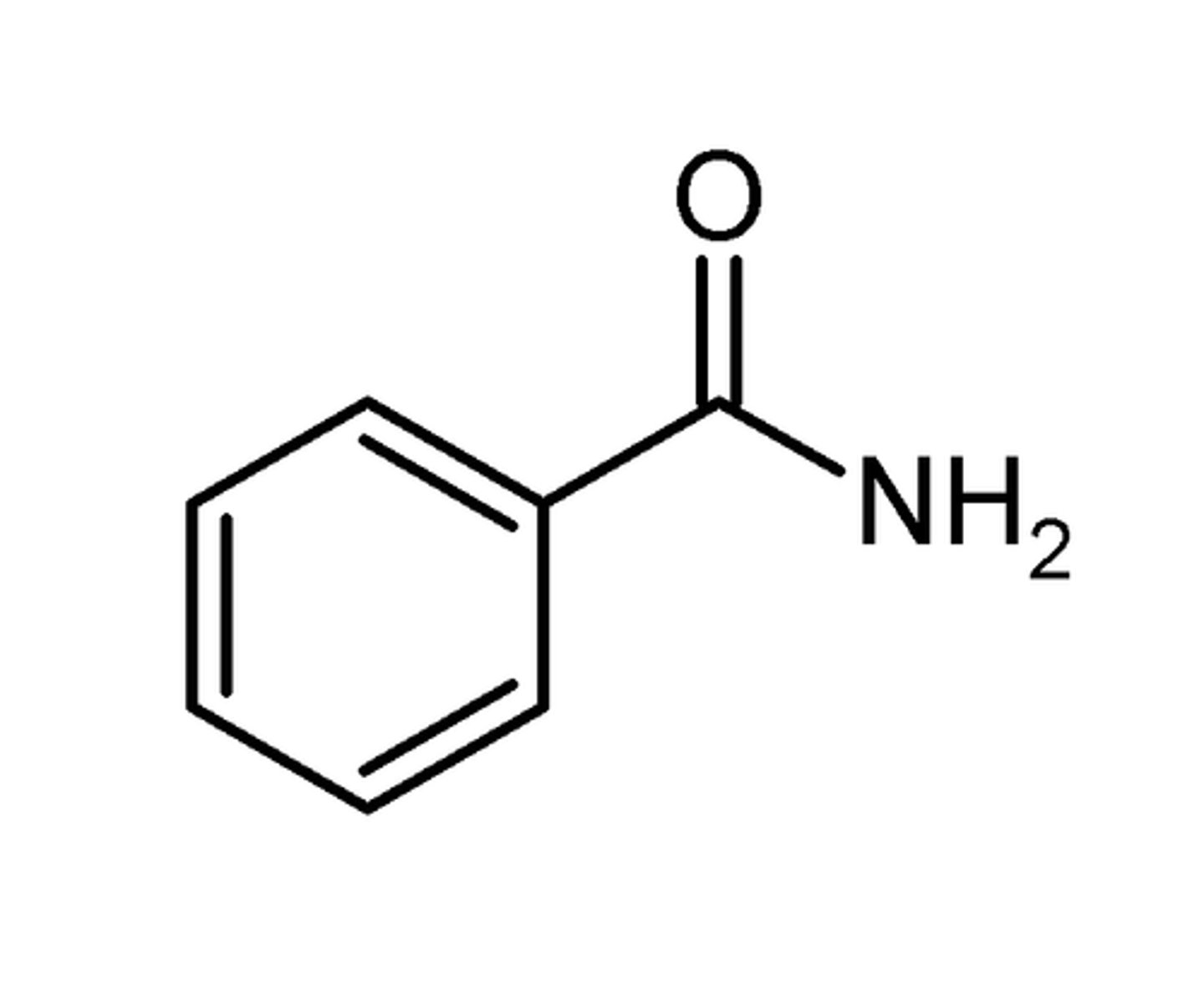
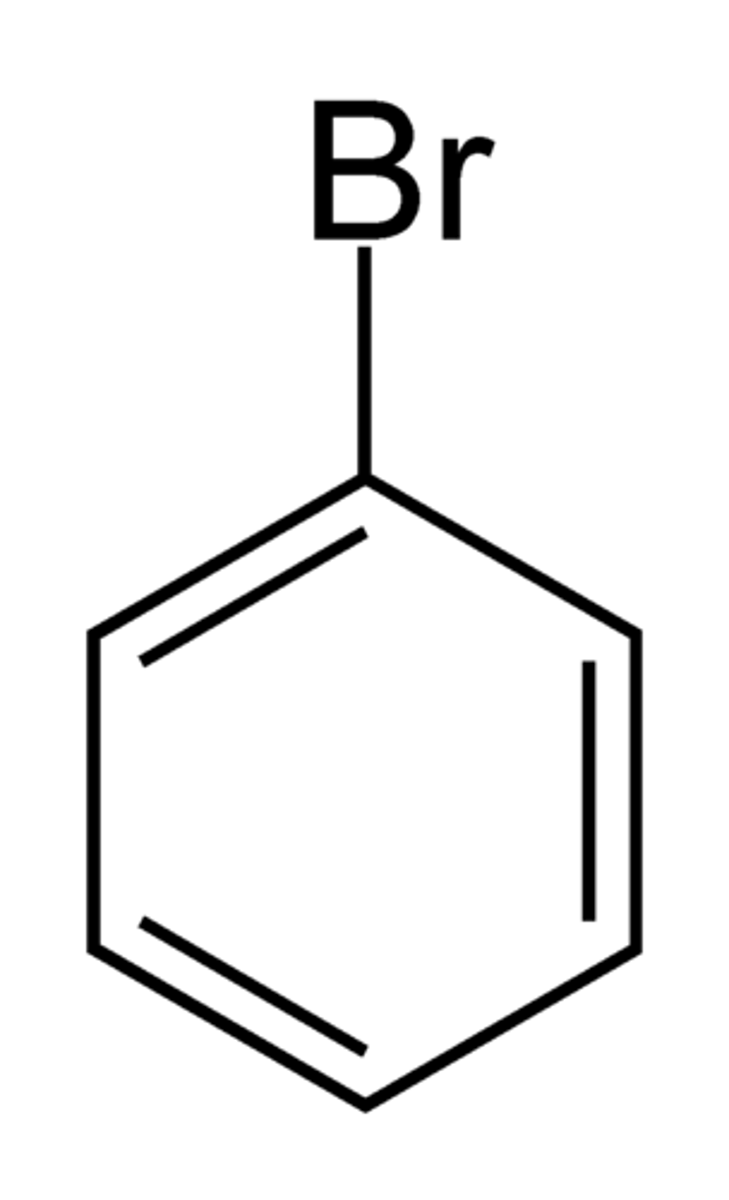
bromobenzene
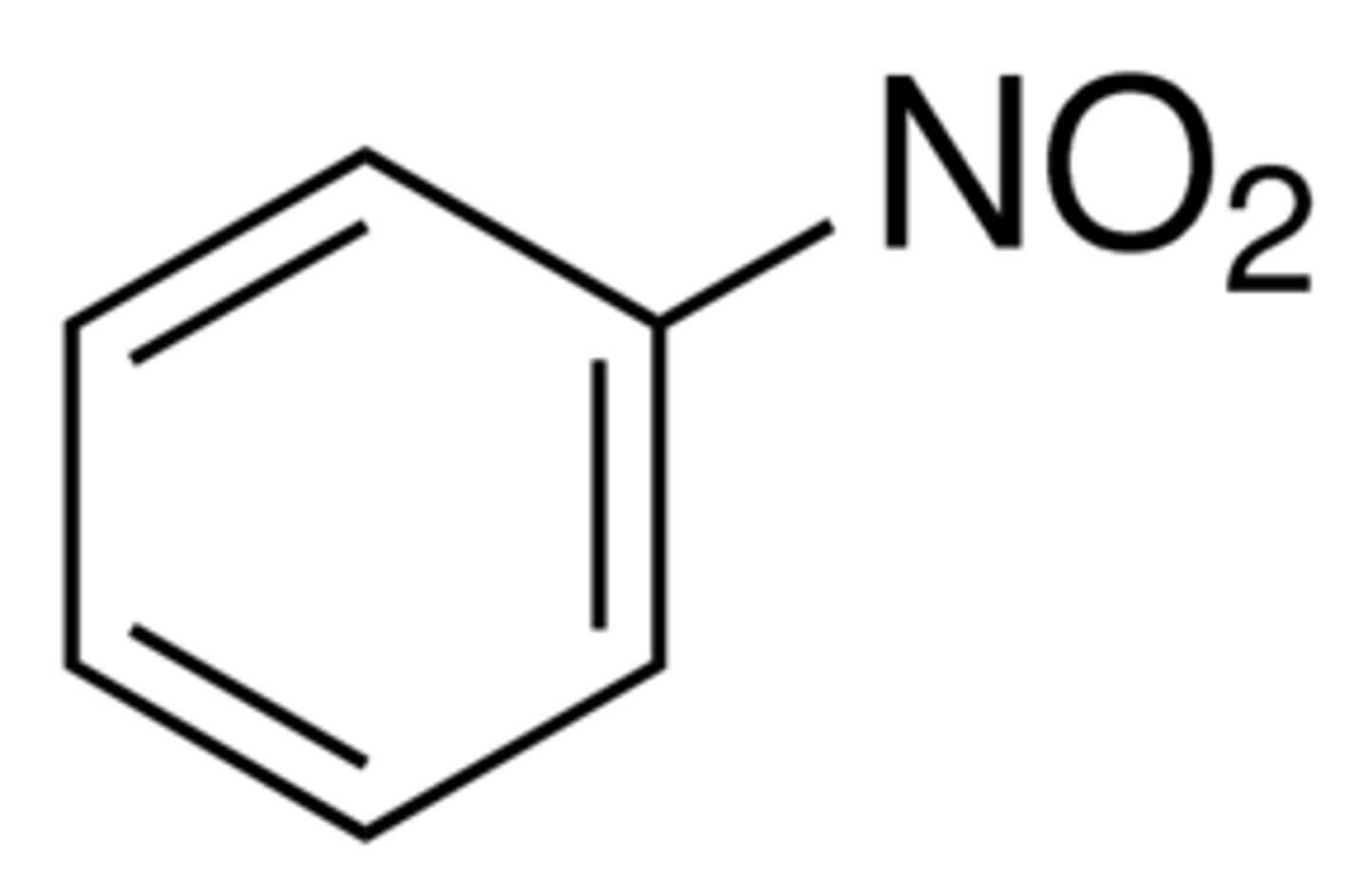
nitrobenzene
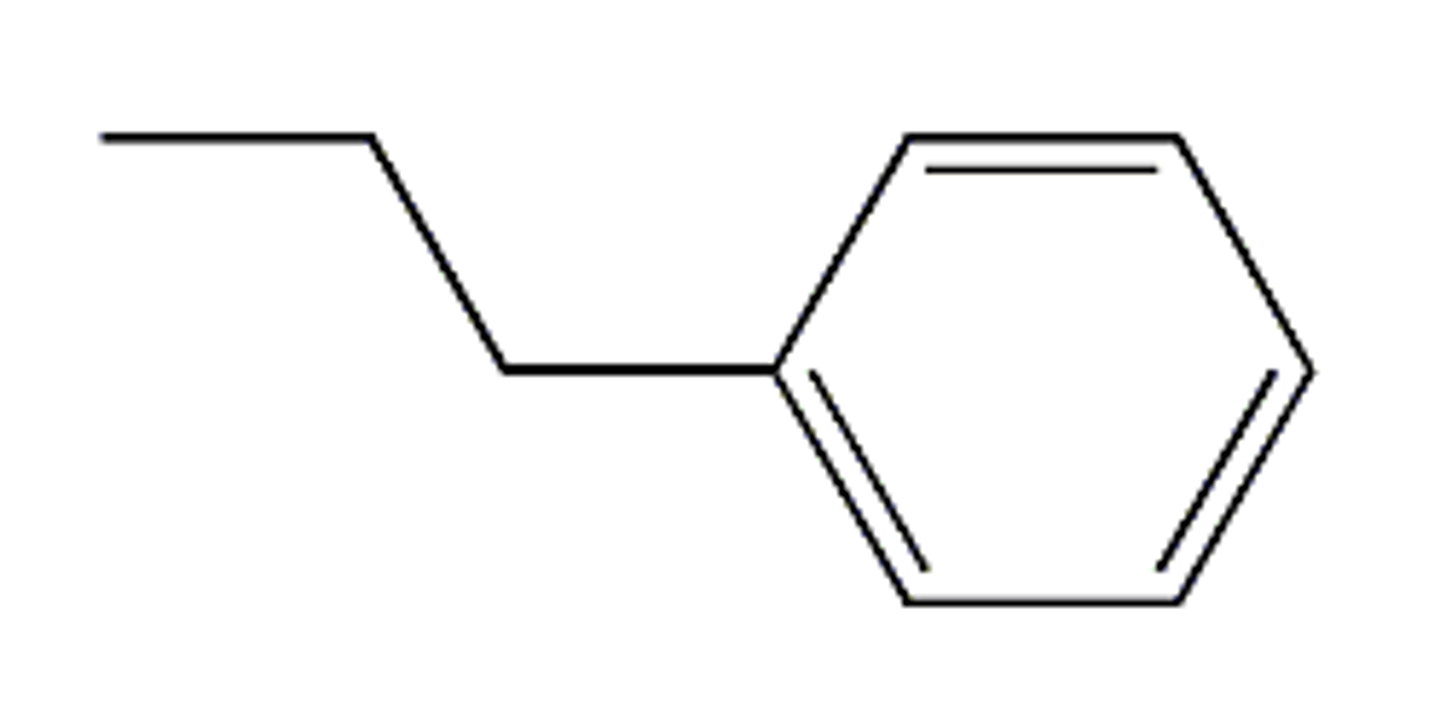
propylbenzene
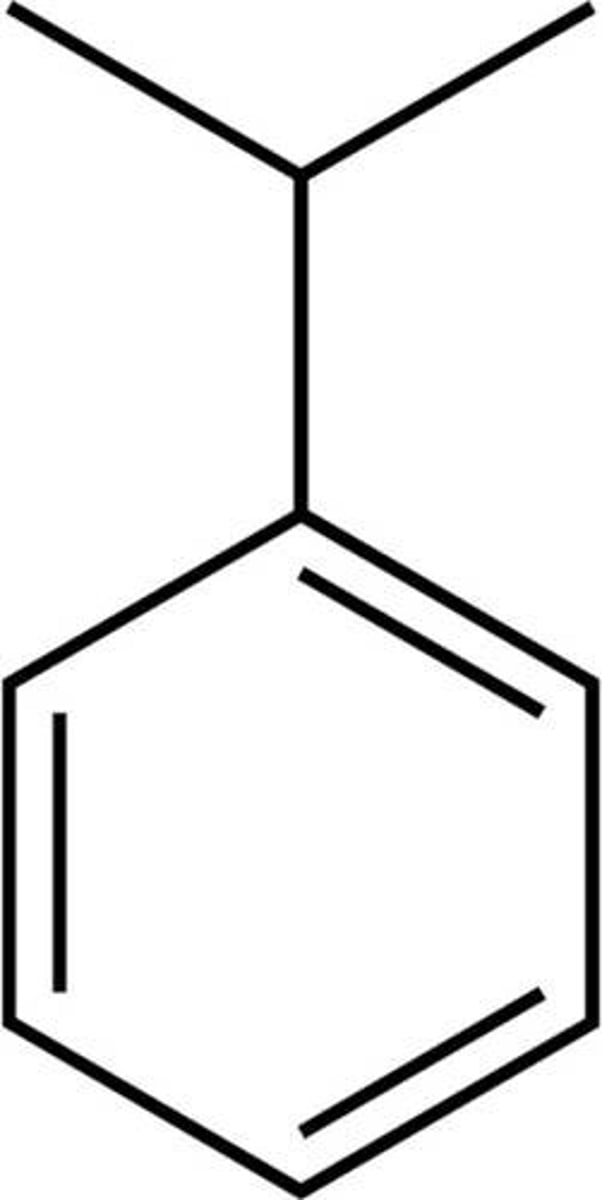
cumene
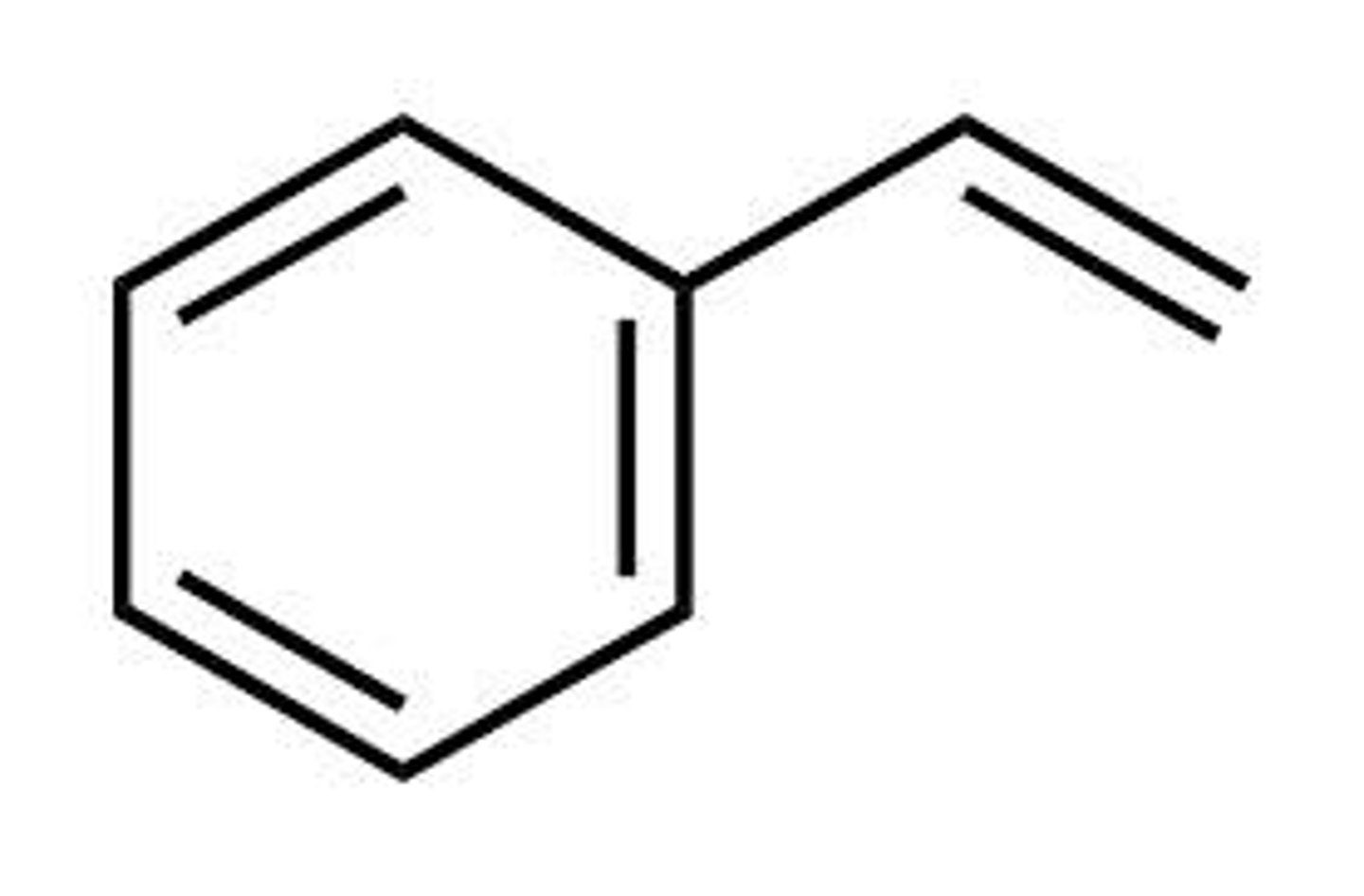
styrene
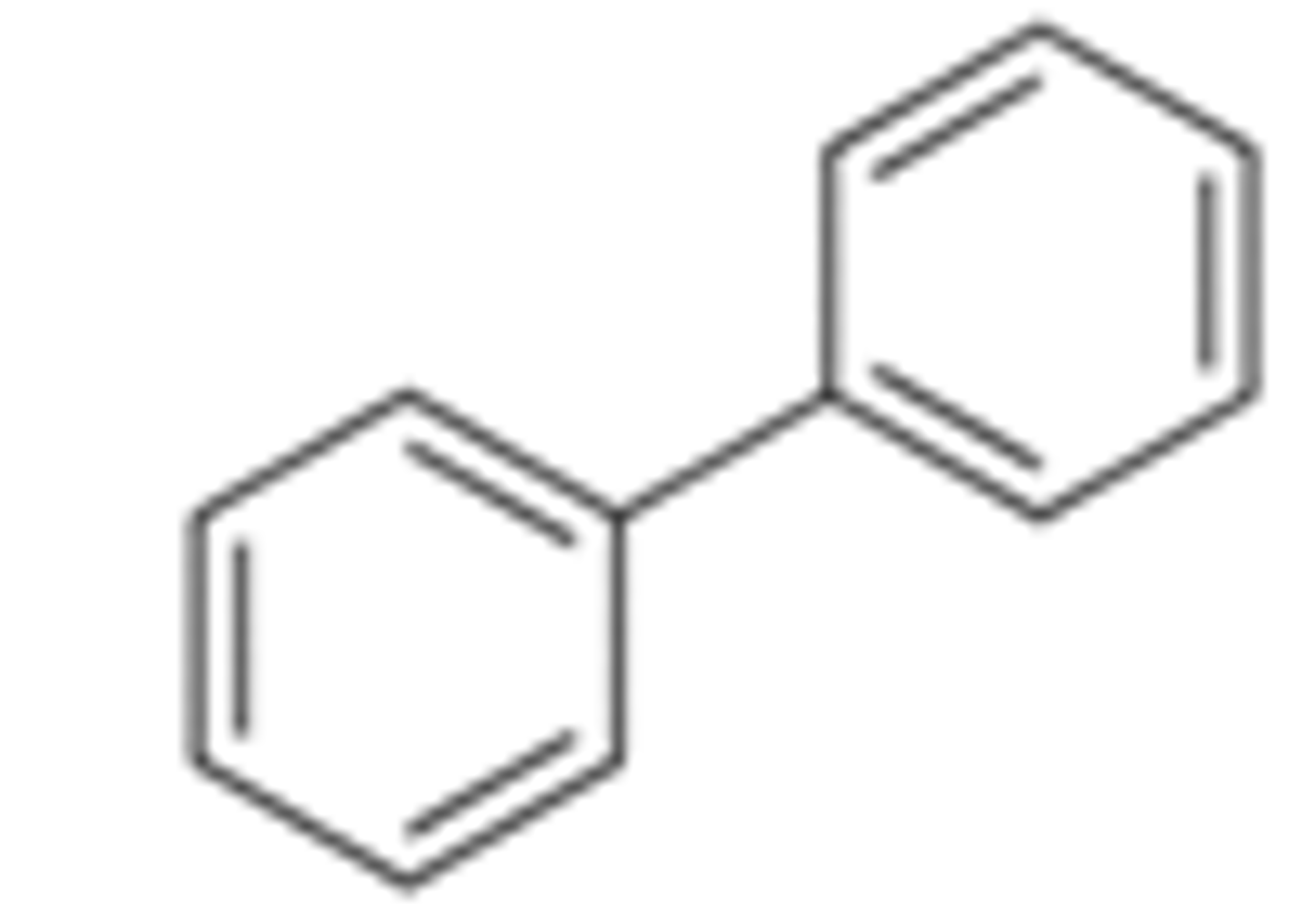
biphenyl
Regioisomer
Structural (constitutional) isomers differing in the position of one substituent
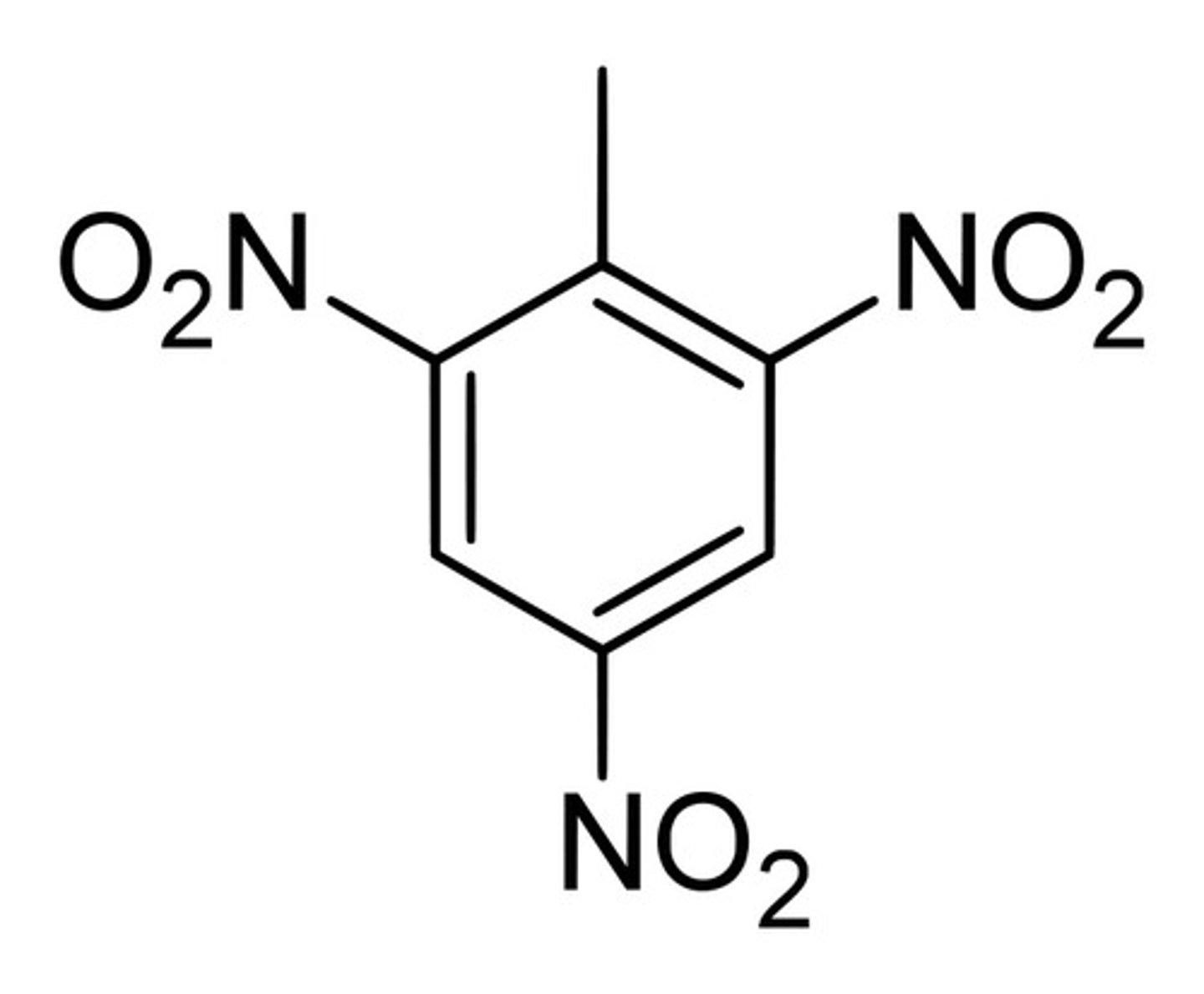
2,4,6-trinitrotoluene
TNT
Requirements for antiaromatic system
Cyclic, planar, fully conjugated and contains (4n) π electrons (n≥1).
(4n) π electrons = 4, 8, 12, 16...
What are antiaromatic systems like?
Destabilised - higher in E
→ very reactive and rather unstable
→ often require isolation at low temp
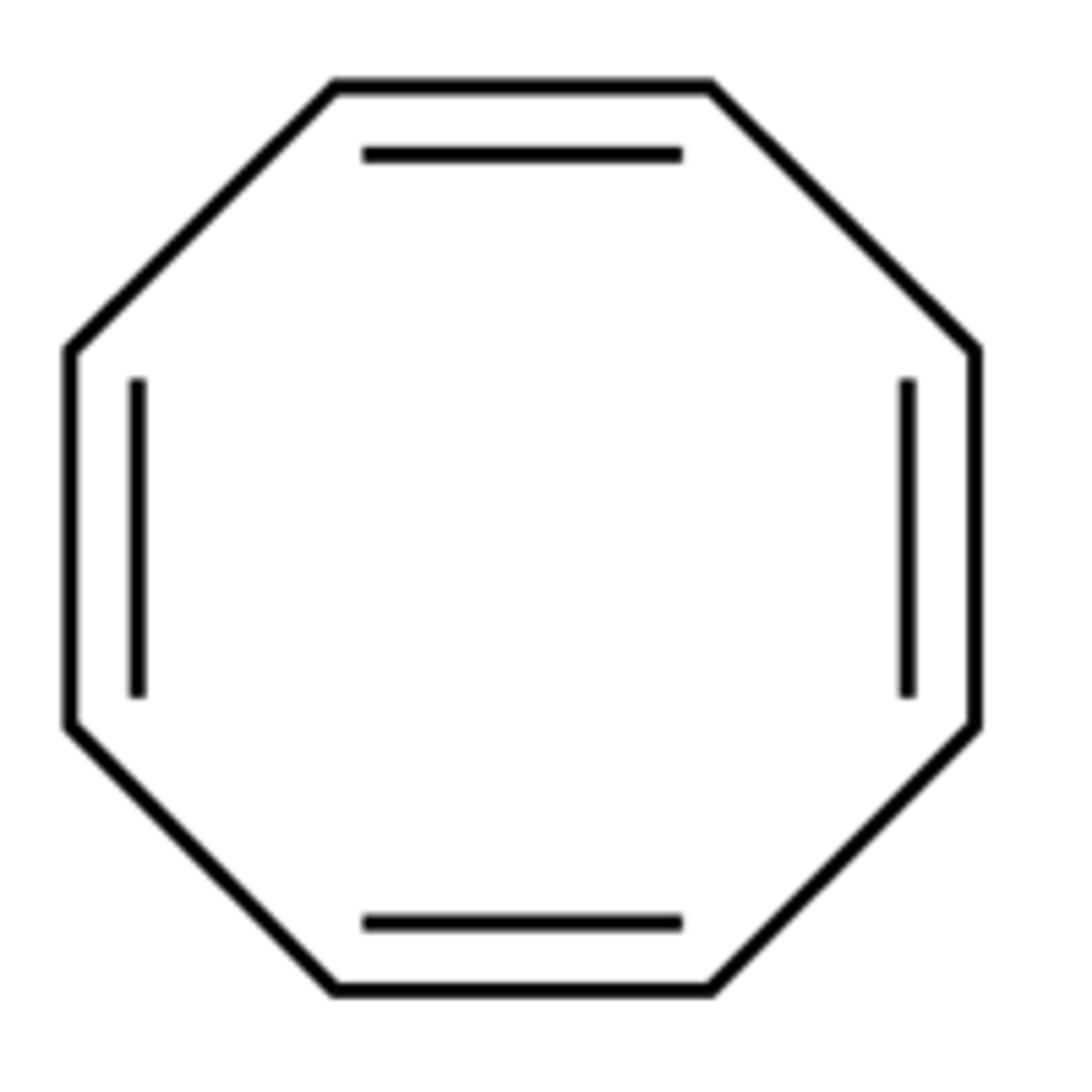
Give an example of an compound that avoids antiaromaticity.
Compounds often try not to be antiaromatic (e.g. by breaking planarity)
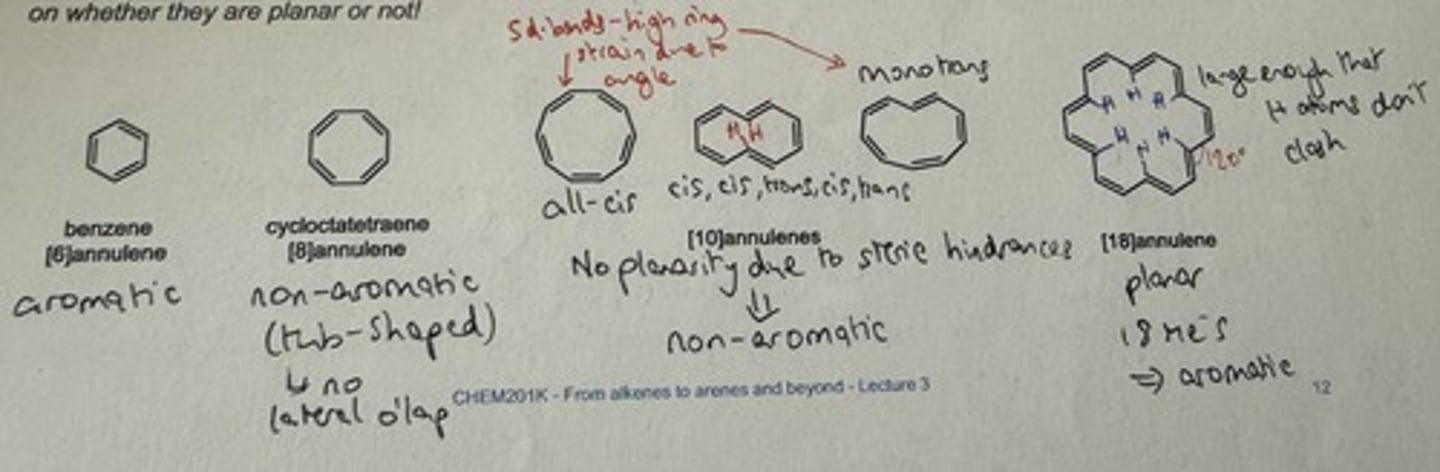
→ cyclooctatetraene - tub-shaped
→ cyclic, 8 pi e⁻s, fully conjugated but is not planar
Give an example of an anti aromatic system.
cyclobutadiene
→ cyclic, planar, fully conjugated, 4n electrons (4, n=1)
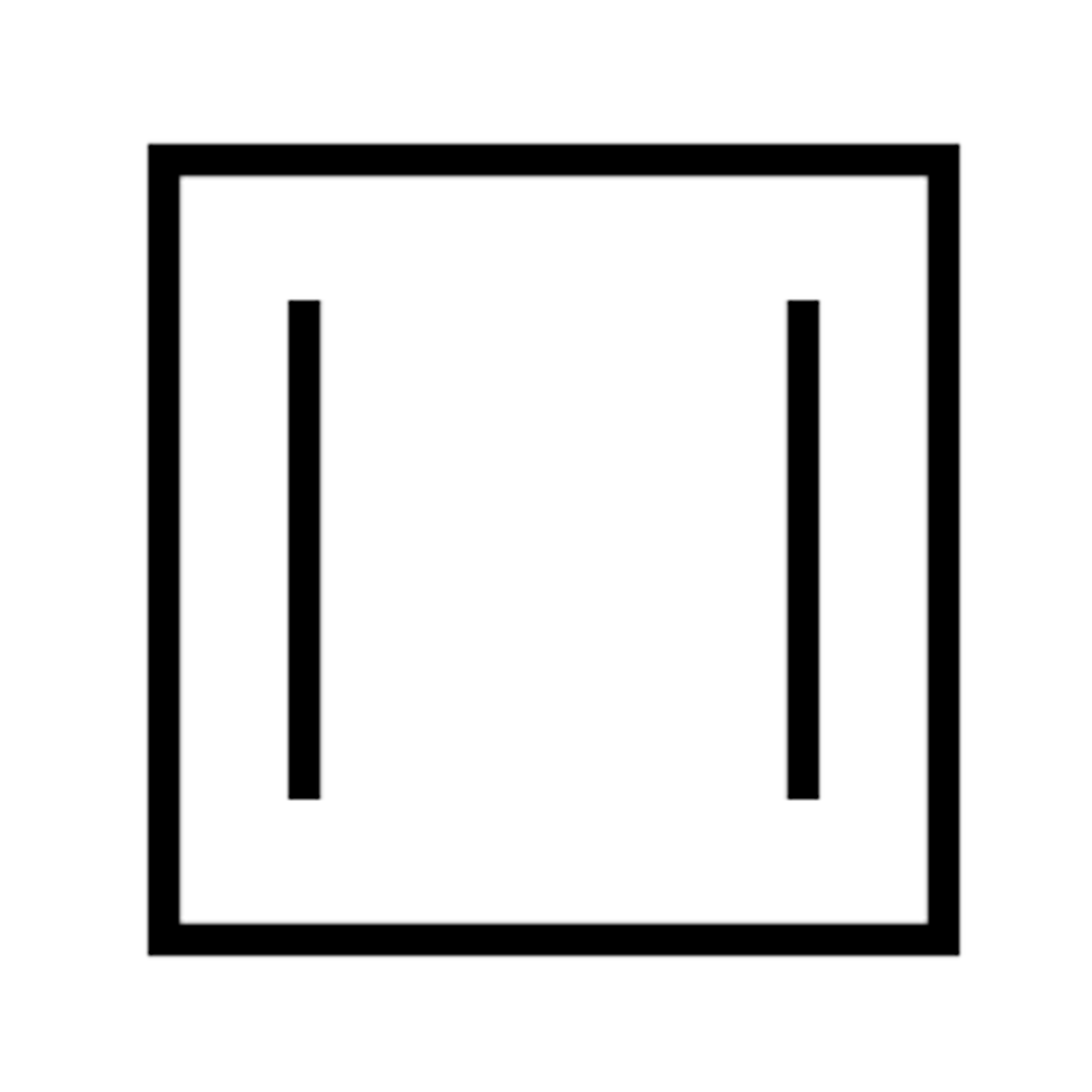
Annulene
Monocyclic hydrocarbon compounds w/ max possible no. of non-cumulated double bonds.
Gen formula: CₙHₙ (if n=even) or CₙHₙ₊₁ (if n=odd)
Can be aromatic, antiaromatic or non-aromatic. Depends on no. of π electrons and whether they're planar or not.
Examples of annulenes
π molecular orbitals for benzene
Has 3 bonding π orbitals, lower in E and completely filled → stabilisation
Show Frost's circle method for drawing π MOs in annulenes (C backbone only)
toluene
methylbenzene!!