APCHEM- unit 5 active recall
1/129
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
130 Terms
How do you find faherinet from celsius?
9/5C+32
What are intermolecular forces?
forces between molecules
what is the equation for pressure?
P=
F/A
-------------
P= pressure in (Pa)
F= force in newtons (N)
A= area in m²
Celsisu to faherint
(F-32) (5/9)
Equation to solve for the number of gas mols produced
n= (PV)/RT
Volume is read, R is known, T is given but P is (1atm-Ph2O)
what is the formula for root means square velocity (Urms)?
... M is molar mass
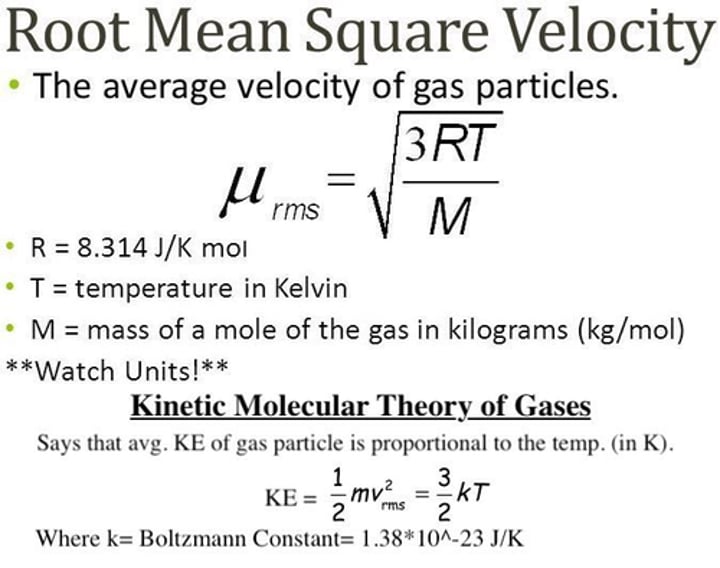
what value of R should you use?
8.314 J/molK
what is the formula for average speed/velocity
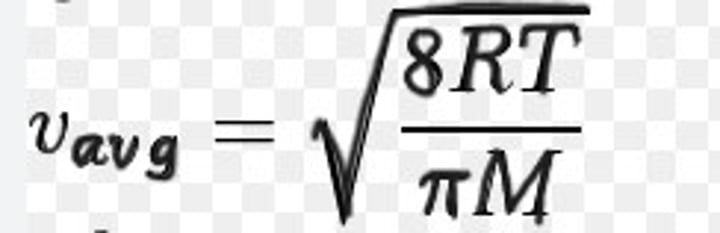
what is the equation for most probable speed?
peak of graph average
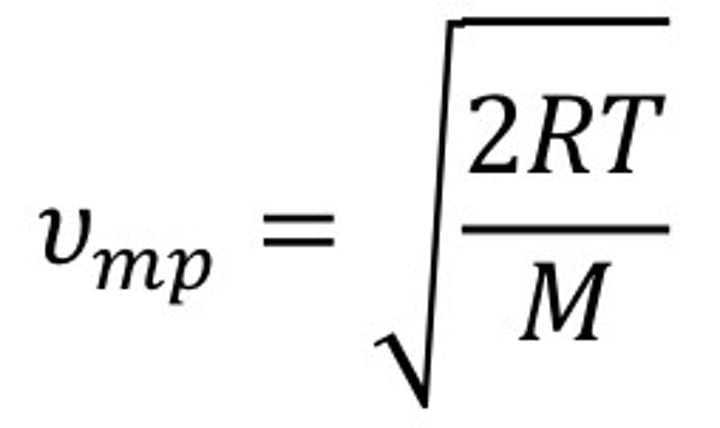
what is the equation for Joules (J)
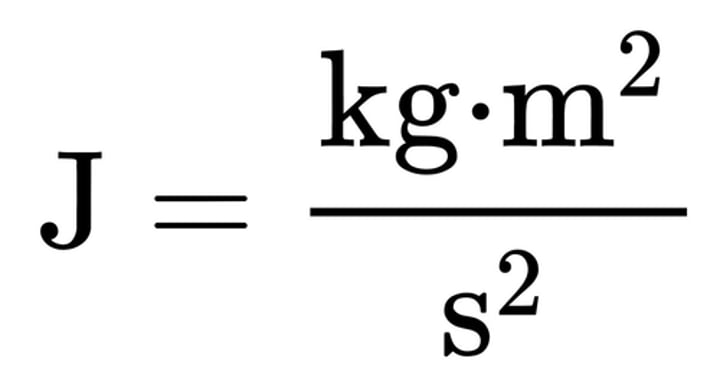
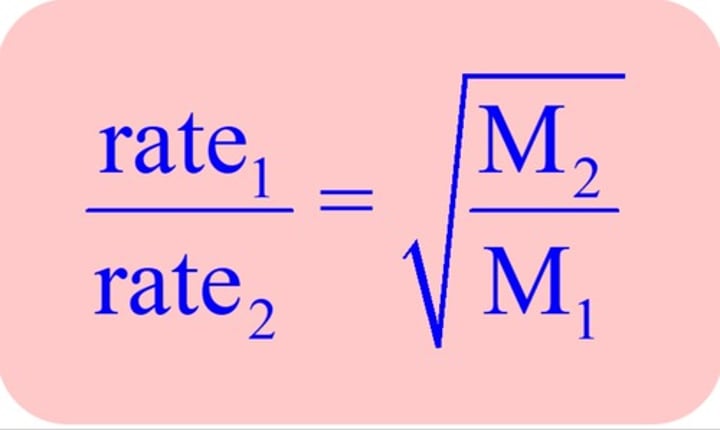
What is Grahm's Law of Effusion equation?
faster / smaller molecules move through faster
Are intermolecular or intramolecular forces stronger?
intramolecular forces (ionic, covalant bonds) are stronger
What are some common intermolecular forces?
-Disperson forces/ London Disperson Forces,
-Dipole-dipole interactions,
-Hydrogen bonding
-Ion-dipole forces
What are Disperson/London dispersion forces?
-temporary attractions between an instantaneous dipole and an induced dipole
-transient event
What causes London dispersion forces?
-constant motion of electrons (partial positive and negative)
-induced dipole is caused by an electron being near it
-instantaneous dipole induces another
what molecules have london disperson forces?
every molecule
how do number of electrons and molecular weight impact london disperson forces?
-The more electrons, the stronger the dipole and is more likely to induce a dipole, stronger partial negatve
-The higher the molecular weight, the stronger
What is a dipole-dipole force?
-The attraction between the positive end of one polar molecule and the negative end of another
What is a dipole?
a molecule with a partial negative charge because of electronegativity differences
Dipole-dipole forces only work for what type of molecules?
Polar molecules
Is CH4 polar?
no, but it is neutral
What do polar molecules have?
permanent dipoles and are ASSYMETRIC
Are dipole-dipole interactions permanent?
yessss
How does electronegativity difference impact dipole-dipole interactions?
Increased difference means stronger force
What is hydrogen bonding?
hydrogen atom that is bonded to a highly electronegative atom (FON) is attracted to an unshared pair of electrons of an electronegative atom in a nearby molecule
-very strong force
what is required for hydrogen bonding?
F,O,N
what does hydrogen bonding cause?
-Weird properties of water like expanding when it freezes bc of the cystalline structure
-also why ice floats, low density
What are ion-dipole forces?
attractive forces between an ion and a polar molecule
-dissociation, ionic substance in polar substance
what is the strongest force?
ion-dipole
what occurs during ion-dipole forces?
the negative or postitve ion is strongly attracted to the pos or neg dipole
what is different about ion-dipole forces?
it is acutally positive or negative while otehr forces are partially, not fullly
what can intermoleular forces predict?
the state of matter
what do higher forces lead to?
higher melting and boiling points, makes it harder for phase changes because melting and boiling causes separation of molecules
-why water boils rlly high
What is viscosity? What are things with high viscosity called?
resistance to flow, viscous
The harder it is to flow...
the higher the viscocity
How does intermolecular forces affect viscocity? How does temperature affect viscocity?
-Higher interforces mean higher viscotity
-Higher the temp, the lower the viscocity
Is viscocity a coehsive or adhesive force?
Cohesive
What does cohesive forces mean?
Occurs between moleculars of the same thing
What does adhesive forces mean?
Occurs between two different things
What is surface tension? Is it an coehsive or adhesive force?
a measure of how hard it is to break the surface of a liquid
-why water puddles and beads up , hydrogen bonding
-cohesive
what molecules have high surface tension?
Hg and H2O
what is required to increase surface area of a liquid?
energy
What is capillary action? coehesive or adhesvie?
the attraction of the surface of a liquid to the surface of a solid,
tendency of polar substances to climb surfaces they are adjacent to
-adhesive force
What is a meniscus caused by?
capalliary action
Phase change: solid to liquid
melting
Phase change: liquid to solid
freezing
Phase change: liquid to gas
evaporation
Phase change: gas to liquid
condensation
Phase change: solid to gas
sublimation
Phase change: gas to solid
deposition
What is the heat/enthalpy of fusion ?
energy required to melt 1 mole of a substance
what is waters ΔHfus?
6.01 kJ/mol
what is heat/enthalpy of vaporization?
energy required to evaporate/boil 1 mole of a substance
what is waters ΔHvap?
40.7 kj/mol
what is heat/enthaply of sublimation?
energy required to sublimate 1 mole of a substance?
what is water ΔHsub?
46.7 kj/mol
heat of fusion + heat of vaporization =
heat of sublimation
here is a heating curve graph
the x axis can also be time
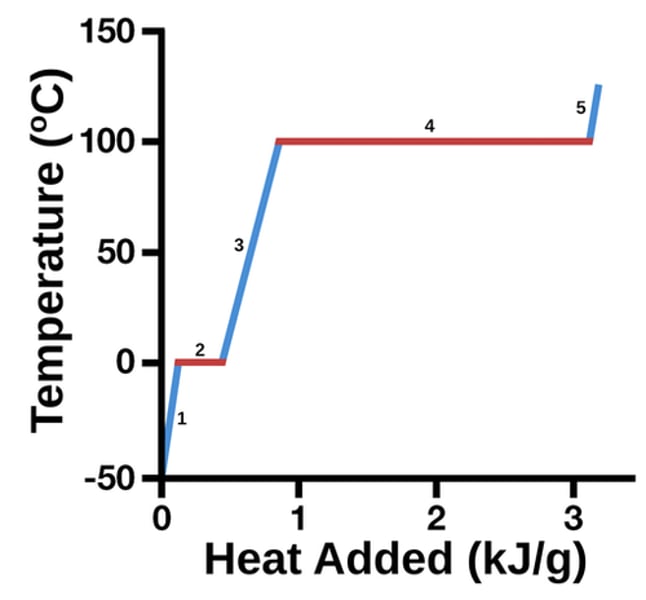
What do the flat parts mean?
phase change is happening
Why is the highest line/ vaporizing line so long?
It takes more energy to go from liquid to gas because it takes more energy to fully separate molecules
how to Hfus and Hvap relate to the chart?
they are the energy required to put in
What are the three heat equations?
q = mcΔT,
q=mΔHfus
q=mΔHvap
when do you use q = mcΔT
temperature changes
when do you use q=mΔHfus or q=mΔHvap?
Hfus, at the melting point and the other at the boiling point
What is the specific heat(C) of water as a liquid?
4.18 J/gk
What is the specific heat(C) of water as a solid?
2.09 J/gk
What is the specific heat(C) of water as a gas?
1.84 J/gk
What is specific heat?
amount of energy needed to raise 1 gram of a substance 1 degree Celsius
Phase diagram
a graph showing the conditions at which a substance exists as a solid, liquid, or vapor
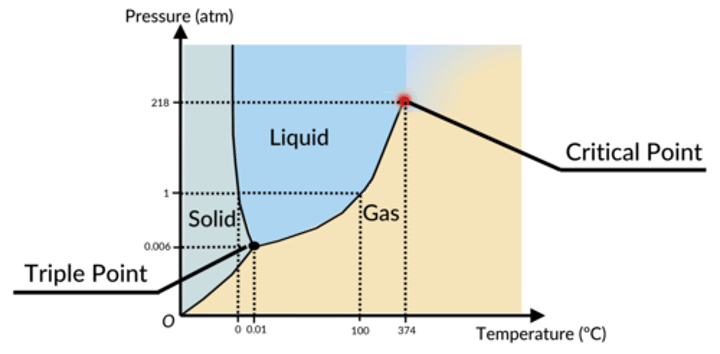
What is the critical temperature?
The highest temperature at which a given substance can be a liquid
what is the critical pressure?
pressure at which the critical temperature substance is still a liquid
what is vapor pressure?
pressure exerted by a liquid when it reaches dynamic equllbrium
what is the supercritical fluid?
any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist
What is the triple point?
the temperature and pressure at which all three phases of matter coexist in an equilibrium
how does temperature impact vapor pressure?
increase in temperature, increase in vapor pressure
what is boiling point?
when vapor pressure equals atmospheric pressure.. lower atm means lower bp... think mountains
how do IMF (intermolecular forces) impact vapor pressure?
the higher the IMF, the lower the VP
high VP means
volatile
Clausius-Clapeyron graph
an equation that displays the exponential relationship between vapor pressure and temperature
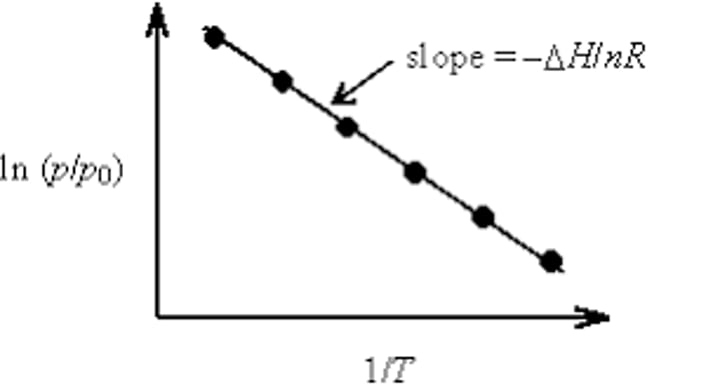
what is the slope equation?
slope= -ΔHvap/ R
What is the Clausius-Clapeyron equation?
ln[(P1/P2)]= (-ΔH/R)*[(1/T2) - (1/T1)]
![<p>ln[(P1/P2)]= (-ΔH/R)*[(1/T2) - (1/T1)]</p>](https://knowt-user-attachments.s3.amazonaws.com/1edbd533-33c0-437c-8009-2591effb6b7c.image/jpeg)
R=
8.314 J/molK
the clausius-clapeyron equation has to be in what?
kelvin
ln sigfigs
ignore anything before the decimal
1 atm=
760 mmHg
760 torr
101.3 kPa
end of chapter 11
ok lets go again
What is the kinetic molecular theory?
based on the idea that particles of matter are always in motion
What are the ideal gas laws?
-Gas molecules are in constant, rapid, straight-line motion
-gas molecule collisions are elastic
-gas molecules have no volume
-gas molecules have no attraction for one another
what do elastic collisions mean?
the gas molecules bounce off each other with the same energy each time with no/negligible lost of energy
what parts of the ideal gas laws are somewhat true or false ?
-gasś do move in rapid straight line motions
-gas kind of have elastic collisions, some energy lost but small
-gas molecules do have some volume but very small amount
-no attraction for one another... just wrong
What are the 5 rules of the kinetic molecular theory?
1. Random motion
2. negliable molecular volume
3. negligible forces
4. constant average kinetic energy
-> some energy can be transferred during collisions but as long as temp is constant, average KE dont change
5. avg KE proportional to temperature
->at any given temp, the molecules of all gases have the same avg KE
what are some true facts about gases?
-no definate shape... takes shape of container
-no definate volume... equal to containers volume: means gas expands indefinitely until container reached
-gases are fluid (move around)
-low density
-form homogenous mixtures
-are compressible
-diffuse and effuse
-exert pressure
what does it mean to form homogenous mixtures?
evently distributed mixture
what are some unique things gases do that solids and liquids dont?
compressible and diffuse and effuse
What does diffusion mean?
movement of particals from high conentration to low concentration, rate gas fills the container
what does effusion mean?
rate at which gas molecules escape a small hole
What is pressure?what is it caused by?
Force per unit area. Caused by collisions
What is the formula for newtons?N=
(kg x m) / s²
s= seconds
What conditions cause more idealish gases?
High temp, low pressure
Standard pressure amounts
1 atm, 760 torr, 760 mm Hg, 101.3 kPa