Topic 1.1 P`articles in the atom and atomic radius
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
Describe the structure of an atom.
An atom consists of a very small, dense nucleus containing protons and neutrons, surrounded by electrons in shells in mostly empty space.
State the relative charge of a proton, neutron and electron.
Proton: +1
Neutron: 0
Electron: −1
State the relative mass of a proton, neutron and electron.
Proton: 1
Neutron: 1
Electron: 1/1836
Define atomic number.
The number of protons in the nucleus of an atom.
Define mass (nucleon) number.
The total number of protons and neutrons in the nucleus.
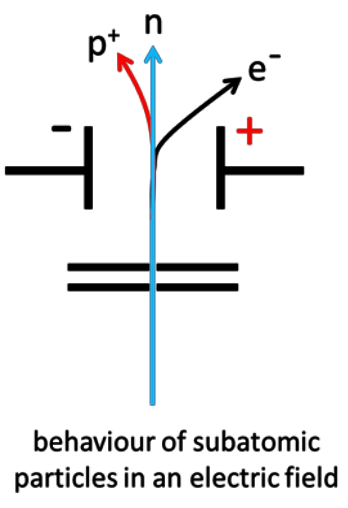
Describe the behaviour of protons, neutrons and electrons in an electric field.
Protons: deflected towards the negative plate
Electrons: deflected towards the positive plate (more strongly)
Neutrons: not deflected
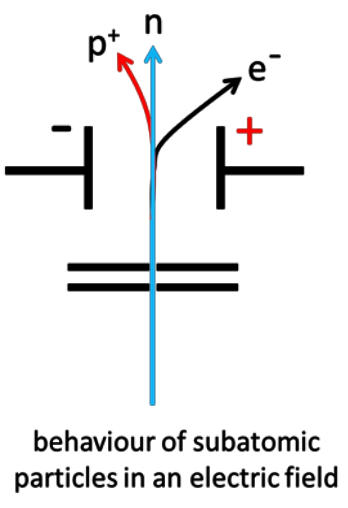
Explain the behaviour of protons, neutrons and electrons in an electric field.
Protons are deflected on a curved path toward the negative plate. Electrons are deflected significantly more on a curved path toward the positive plate due to the low mass. Neutrons continue in a straight line as they are not attracted to either plate.
In an electric field, do protons, electrons and neutrons travel at the same velocity or different velocity?
Same velocity
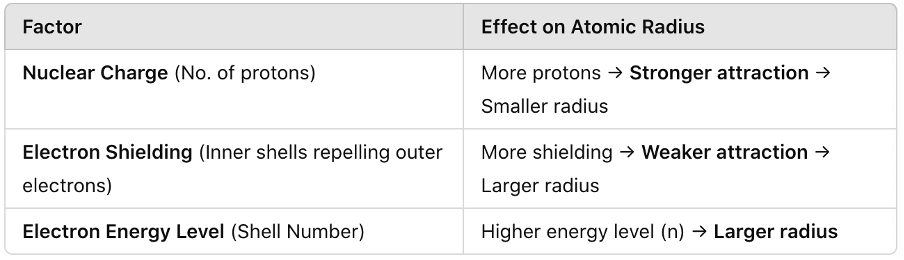
State and explain the trend in atomic radius across a period.
Atomic radius decreases across a period due to increasing nuclear charge with electrons added to the same shell.
State and explain the trend in atomic radius down a group.
Atomic radius increases down a group due to additional electron shells and increased shielding.
Describe the distribution of charge in an atom.
Positive charge is in the nucleus; negative charge is in the surrounding electrons.
Describe the distribution of mass in an atom.
Almost all the mass is concentrated in the nucleus.
Define ionic radius.
The radius of an ion in a crystal lattice.
How does the radius of a positive ion compare to its atom?
A positive ion is smaller than its atom.
Explain why a positive ion is smaller than its atom.
Loss of one or more electrons reduces electron–electron repulsion and increases attraction between the nucleus and remaining electrons.
How does the radius of a negative ion compare to its atom?
A negative ion is larger than its atom.
Explain why a negative ion is larger than its atom.
Gain of electrons increases electron–electron repulsion and reduces the attraction per electron.
Write in order of smallest to largest: Anion, Cation, Atom
Cation, Atom, Anion
Cation charge
positive (think cat)
Anion charge
negative (think onion)
Describe the trend in ionic radius across a period for positive ions.
Ionic radius decreases across a period for positive ions (cations).
Explain the decrease in ionic radius across a period for positive ions.
Increasing nuclear charge with the same number of electron shells. There is a stronger force of attraction between the nucleus and outer shell electron.
Describe the trend in ionic radius down a group.
Ionic radius increases down a group.
Explain the increase in ionic radius down a group.
Additional electron shells and increased shielding.
Define isoelectronic species.
Species with the same number of electrons and the same electronic configuration.
Compare the ionic radii of O²⁻, F⁻, Na⁺ and Mg²⁺.
O²⁻ > F⁻ > Na⁺ > Mg²⁺
Explain the trend in ionic radius in an isoelectronic series.
Increasing nuclear charge pulls electrons closer to the nucleus
Describe the trend in ionic radius across a period for negative ions.
Increases across a period for negative ions (anions)
Explain the trend in ionic radius across a period for negative ions.
Negative ions (anions) increase in size as they gain more electrons (interelectronic repulsion causes the electrons to spread out) with no increase in nuclear charge
What factors affect atomic radius?
"Explain why the atomic radius of chlorine (Ar=17) is smaller than that of sodium (Ar=11)."
Chlorine has more protons (17) than sodium (11) → Greater nuclear attraction.
Both elements have the same number of electron shells → No extra shielding effect.
Stronger attraction pulls outer electrons closer → Smaller radius.
Compare the atomic radii of lithium (Li) and caesium (Cs). Explain your answer.
Cs has a larger atomic radius than Li.
Reason:
Cs is lower in Group 1, meaning it has more electron shells (n = 6 for Cs vs. n = 2 for Li).
More shielding from inner electrons reduces nuclear attraction.
Weaker attraction between nucleus and outermost electron allows a larger atomic radius.
Trend: Down a group, atomic radius increases.
Compare the atomic radius of Na and the ionic radius of Na⁺. Which is larger and why?
Na has a larger atomic radius than Na⁺.
Reason:
Na⁺ loses an electron, meaning one fewer energy level (shell), so the electron cloud is pulled closer.
Greater proton-to-electron ratio = Stronger attraction pulling the remaining electrons closer.
Cations are always smaller than their neutral atoms.