Glycogen Degradation
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Blood Glucose
Energy source that enters through diet and liver from glycogen breakdown or gluconeogenesis
Catabolism
Stored Glycogen
Energy source where liver uses glucose residues in this to replenish blood glucose
Muscle uses glucose residues in this for catabolism and to generate ATP
Stored Triacylglycerides in Adipose Tissue
Energy source that is mobilized mostly to muscle and liver for catabolism
Amino acids — surplus, diet, protein breakdown
Common mechanism for cells to store excess metabolites
Polymerization into large macromolecular structures (polyphosphate, polyhydroxyalkanoate)
Liberation of Glucose from Glycogen Step 1
Release of Glucose-1-Phosphate
Liberation of Glucose from Glycogen Step 2
Remodeling to permit further degradation
Liberation of Glucose from Glycogen Step 3
Conversion of Glucose-1-Phosphate to Glucose-6-Phosphate
Glycogen Phosphorylase
Phosphorolysis of glycogen is catalyzed by this allosteric enzyme
Glycogen Breakdown
Uses phosphate, not water
Glucose residue removed one by one from NON-REDUCING end
Retains configuration at anomeric carbon
Glycogen Phosphorolysis
Yields glucose-1-phosphate; must prevent hydrolysis of glycogen
No energy cost to feed into glycolysis
Phosphorylation glucose stays in cell
Phosphoglucomutase
Exchanges phosphates between a serine residue within its own structure and a glucose-1-phosphate
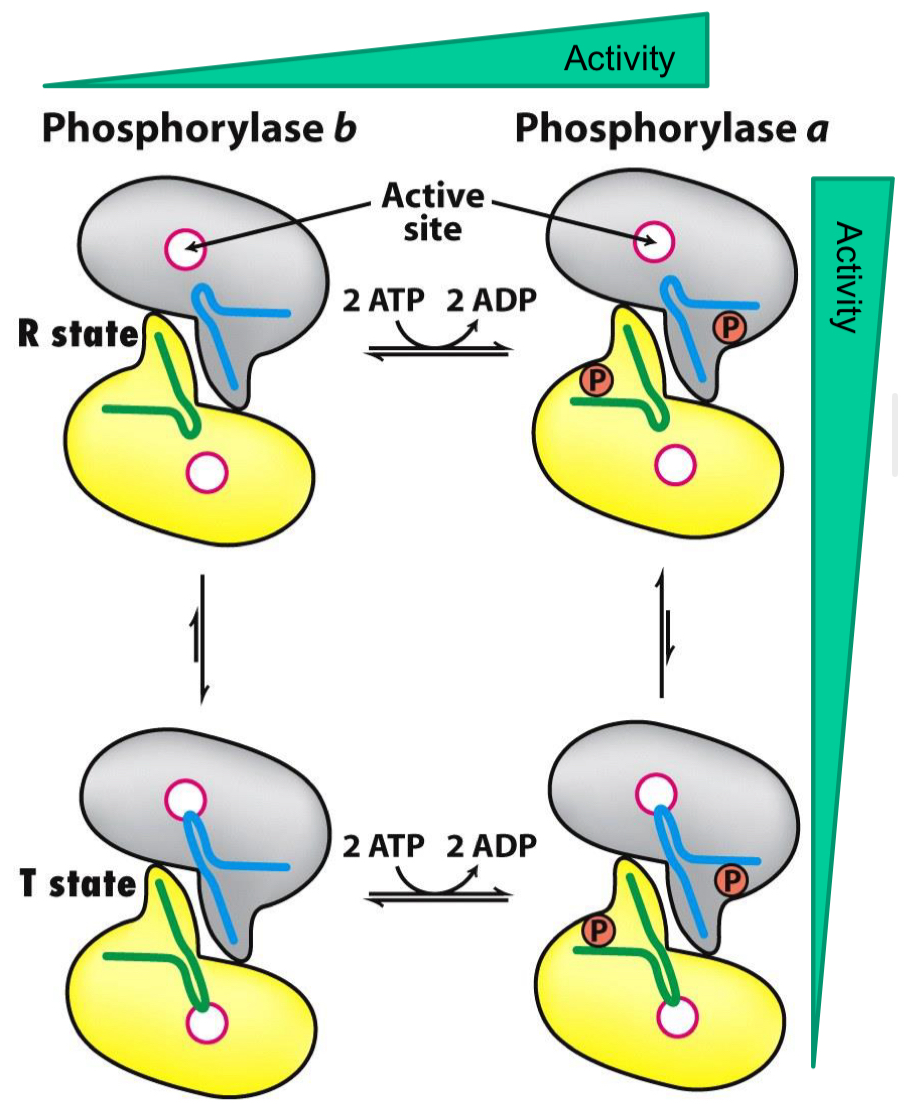
Phosphorylase A
Glucose binding shifts the enzyme from the active form (R-state) to the less active form (T-state)
Phosphorylase B
Activated by high AMP
Binds to a nucleotide binding site and stabilizes the R-state confirmation
ATP and G6P act as negative allosteric effectors
Liver vs Muscle Cells
Standard states of the two cell types are opposite
Leads to different resting activities and different direction of regulation
High [AMP]
Sign that muscle cells have low [ATP] and needs to carbonize glucose
Active T-state binding
Phosphorylase Kinase Dual Control
Ca2+ Activation — Nerve impulse, muscle contraction, hormones
Partly Active
Protein Kinase A (Epinephrine) — Hormones
Fully Active
Activated glucose
Glycogen synthesis requires this for greater control
Follow a separate pathway from degradation (even though it is the exact reverse reactions)
Glycosyl-Enzyme Intermediate
Transferase that can carry the transferred oligosaccharide to its new position before the second nucleophilic attack
Glycogen Synthase
Can only work on existing polymers
Required priming with small oligosaccharides
Accomplished by glycogenin dimer
When glycogenin dimer comes together → cross-glycolysation builds up to an 8-sugar primer on opposing monomer
This enzyme then takes over
1 ATP
Cost to regenerate UTP for the activation of glucose
Rest of synthesis does NOT require energy input
More Active
Non-phosphorylase form of glycogen synthase
Phosphorylase B
This enzyme dampens the rate of breaking down glycogen into G1P
Phosphorylase A
This enzyme stimulates glycogen synthesis
Glycogen Synthase A
Is always active when dephosphorylated
Glycogen Synthase B
Is always active when phosphorylated