A: Unity and Diversity
1/435
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
436 Terms
Covalent bond
A bond where two atoms share one or more pairs of electrons
Polar covalent bond
The sharing of electrons is unequal, the oxygen is more attractive to the electrons than the hydrogen. The hydrogen is slightly positive, and the oxygen is slightly negative
Hydrogen bond
The attraction between two polar molecules where the slightly negative atom is attracted to the slightly positive atom by weak electrostatic forces
Cohesion
The binding together of two molecules of the same type
Adhesion
The binding together of different types of molecules
Tension
A pulling force created by the cohesion of water molecules
Surface tension
The property of the surface of a liquid that allows it to resist an external force due to the cohesive nature of the water molecules
Metabolism
The enzyme catalysed reactions that take place in a cell or living organism
Buoyancy
The upward force exerted by a fluid that opposes the weight of a partially or fully immersed object
Polarity of water molecules:
Water molecules are slightly polar
Why are water molecules slightly polar?
Due to uneven distribution of electrons: one side is slightly more positive and the other is slightly more negative
Emergent properties of water:
High specific heat capacity; solvent; cohesive; adhesive
Why does water have a high specific heat capacity?
Lot of energy is needed to overcome many hydrogen bonds. Water requires a high energy input to increase in temperature.
Advantages of high specific heat capacity of water
Water inside living organisms allows them to maintain a stable temperature. Water provides a stable habitat for aquatic organisms.
Why is water a good solvent?
The polarity of water means it is a good solvent for other charged/polar substances
Advantages of water as a good solvent? (Example)
Water is effective for transporting polar molecules (eg. Glucose in blood)
Why is water cohesive?
Polarity of water results in hydrogen bonds between oxygen of one molecule and hydrogen of another molecule. This sticks water molecules together.
Advantages of water being cohesive:
Water sticks together in continuous columns to transport water and minerals in xylem.
Surface tension example
Cohesive forces cause surface tension. The insect’s waxy cuticle prevents the wetting of the body (eg. Raft spider). Mass isn’t great enough to break the surface tension.
Why is water adhesive?
Polarity of water means that it is attracted to other polar substances. Water forms hydrogen bonds with these substances and sticks to them
Examples of adhesion with water (soil)?
Soil contains thin tube, when water enters the tubes, adhesion between water and capillary walls draw water up the tube.
Examples of adhesion with water (plant cell walls)?
Plant cell walls are made of cellulose which is polar and attracts water. Draws water into cell wall from xylem vessel by adhesion.
Examples of adhesion with evaporation from leaves?
When water evaporates from the leaves, it’s replaced by suction forces from the xylem.
Hydrophilic molecules:
Are attractive to water and can form intermolecular bonds with water. They can dissolve in water.
Hydrophobic molecules:
Aren’t attractive to water as water molecules are more strongly attracted to each other instead. They are insoluble in water.
Ions (sodium, chloride, nitrates, magnesium)
Hydrophilic. Sodium ions are positively charged and chlorine ions are negatively charged. Dissolves in the plasma. Dissolves and transported in xylem
Amino acids charge?
Have both negative and positive charges therefore are soluble in water. Solubility depends on the variable part of the molecule (hydrophilic or hydrophobic)
Amino acids (solubility in blood and plants)
Dissolves in plasma. Dissolved and transported in the phloem
Glucose:
Hydrophilic as it is freely soluble in water. Polar molecule. Dissolves in plasma
Sucrose:
Hydrophilic and dissolves in water. Dissolved and transported in the phloem.
Oxygen
Hydrophobic. Non-polar. Hardly dissolves in water (some molecules dissolve as they are so tiny)
Fat molecules
Hydrophobic. Non-polar. Carried in lipoprotein complexes.
What are fat molecules surrounded by?
Layer of amphipathic phospholipids with integral proteins between them
Cholesterol?
Hydrophobic. Carried in lipoprotein complexes between the phospholipids with the hydrophilic region pointing outwards towards the plasma and its hydrophobic regions inwards towards the fat molecules.
Water compared to Air (thermal conductivity)
Water has a higher thermal conductivity than air
Water compared to Air (specific heat capacity)
Water has a higher specific heat capacity than air
Water compared to Air (density)
Water is more dense than air so it provides greater buoyancy than air
Water compared to Air (viscosity)
Water is more viscous and creates greater resistance than air
High thermal conductivity definition:
Heat passes through water more quickly than insulating substances
High viscosity definition:
Hydrogen bonds between water molecules create internal friction when they move against each other and against other surfaces
High buoyancy force:
Water is much denser than air so it exerts a greater upwards force on living organisms which have an overall similar density to water.
Black throated loon:
Type of bird that flies mostly but also can swim on surface of water
Does the loon or the seal use more energy to stay afloat whilst travelling?
Loon uses more energy because air is less dense than water so there is less buoyancy it will need to expend energy to stay in the sky.
Does the seal or loon use more energy to move through their transport medium?
The seal uses more energy as water is more viscous so it takes more energy to overcome the drag created when moving through water.
Does the seal or loon lose more heat energy whilst travelling
The seal loses more heat energy because water conducts heat energy away from the body more efficiently than air
Does the seal or loon experience greater fluctuation in temperature whilst travelling?
Air has a lower specific heat capacity so the loon experiences greater fluctuations in temperature as there is greater changes in temperature in air than in water.
Is thermoregulation a challenge for the loon or the seal?
The loon as it must be adapted to both retaining heat energy when it is cold and losing heat energy produced through flight muscle contraction when it is warm.
Importance of water:
Medium of life; structure of cells relies on water; metabolic reactions are reactions between solutes dissolved in water; liquid water is essential to all known life forms on Earth
How is water retained on Earth?
1) The temperature of Earth means that water stays in a liquid state.
2) Hydrogen bonds in liquid water help to keep it together and stop it from escaping.
3) Earth is large so its gravitational force helps to retain the liquid water & water vapour in atmosphere
What is Goldilocks zone?
The habitable region around a star that isn’t too hot/cold so water remains a liquid
Hypothesis for origin of water on Earth?
1) Collision of asteroids with Earth that has fallen as carbonaceous chondrite meteorites.
2) Heating of these asteroids.
3) Water-ice in these asteroids melts
4) Reaction of hydrogen in these meteorites to form water when asteroids vaporised
5) Water remain on the surface of Earth and became oceans
Viscosity meaning:
The resistance of a liquid to flow created by the internal friction of a moving liquid
State the two primary functions of nucleic acids.
A1.2.1— DNA as the genetic material of all living organisms.
1. Pass genetic information between generations.
2. Code for protein production.
State the two types of nucleic acids used in cells.
A1.2.1— DNA as the genetic material of all living organisms.
DNA: deoxyribonucleic acid
RNA: ribonucleic acid
Outline the meaning and implication of DNA being the genetic material of all living organisms.
A1.2.1— DNA as the genetic material of all living organisms.
Meaning:
All living organisms use DNA as the genetic material.
Implication:
The use of the genetic code across all forms of life is evidence of universal common ancestry of life. The sequences of DNA in cells can be analyzed and compared to determine evolutionary relationships between organisms. The more similar the sequence, the more closely related the organisms.
State why RNA viruses do not falsify the claim that all living things use DNA as the genetic material.
A1.2.1— DNA as the genetic material of all living organisms.
Some viruses use RNA as their genetic material. However, because viruses are not made of cells, they are not considered to be living.
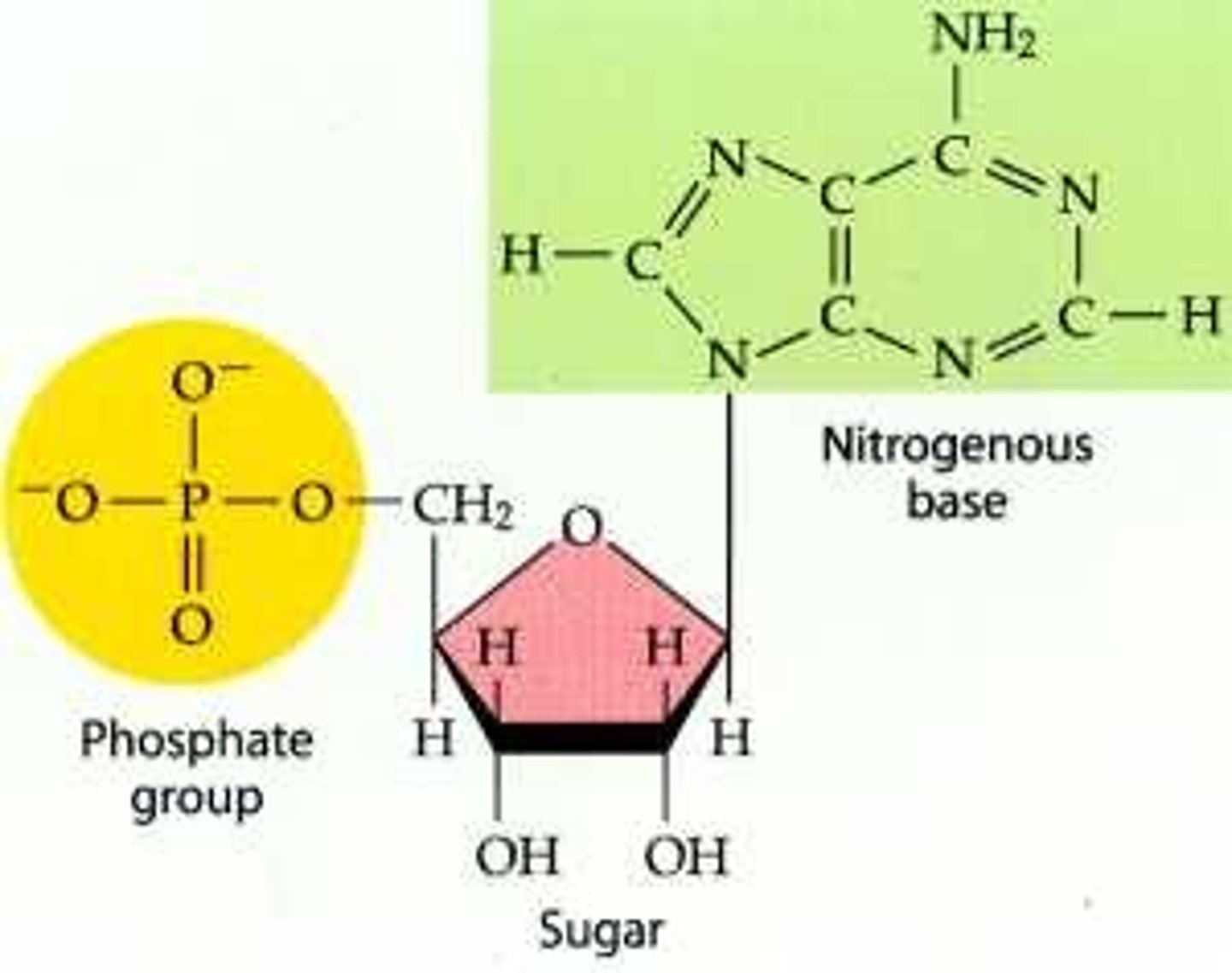
List the three components of a nucleotide.
A1.2.2— Components of a nucleotide.
A nucleotide is the monomer subunit of the nucleic acids. A nucleotide has three component parts:
1. a nitrogenous base
2. A 5-carbon "pentose: sugar (ribose or deoxyribose)
3. A phosphate group
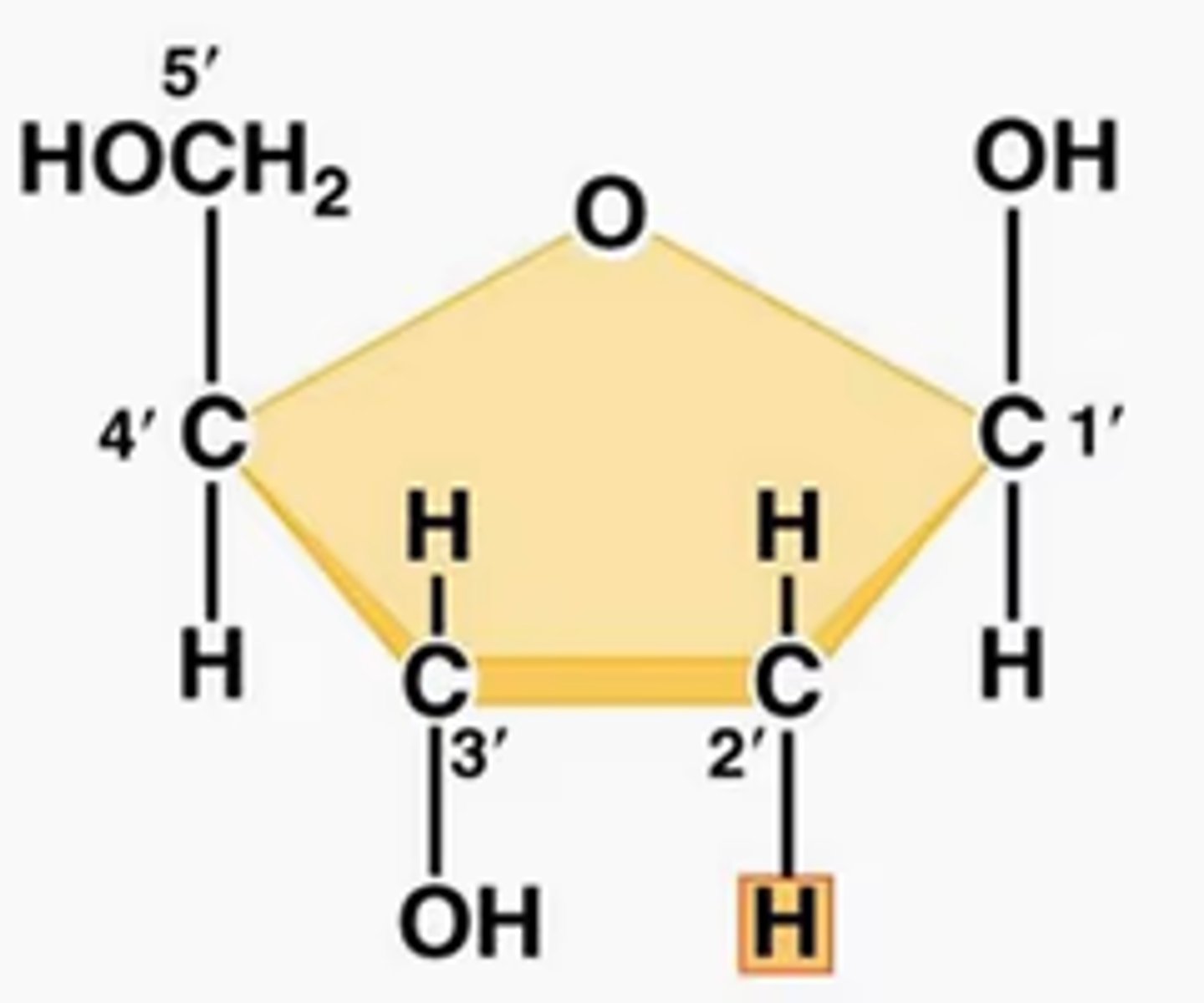
Identify and label the carbons of a pentose sugar.
A1.2.2— Components of a nucleotide.
The carbons of the sugar component of the nucleotide are numbers clockwise, starting from the oxygen in the ring at the top and the phosphate group to the left.
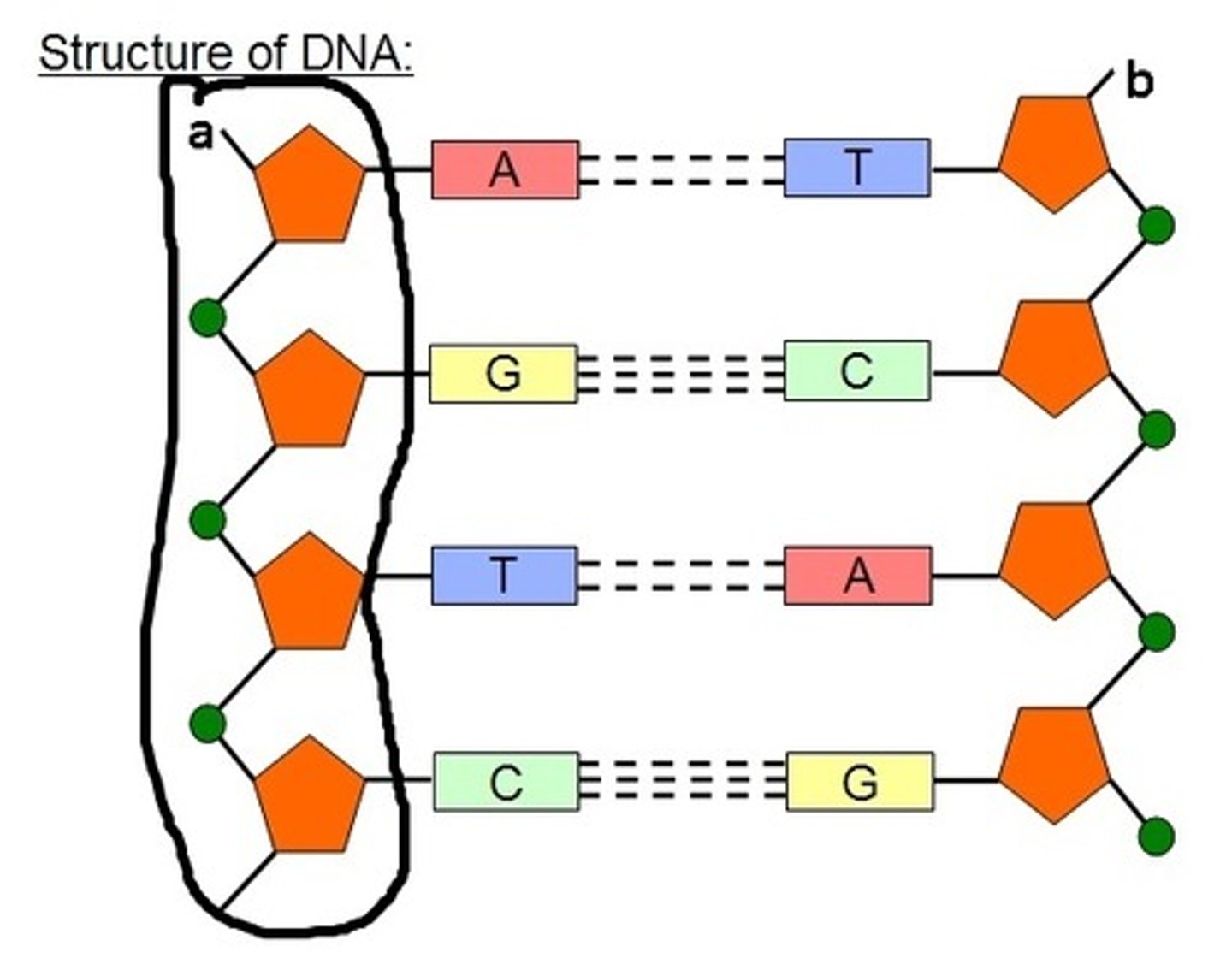
Define “backbone” as related to nucleic acid structure.
A1.2.3— Sugar–phosphate bonding and the sugar–phosphate “backbone” of DNA and RNA.
The “backbone” is the alternating phosphate-sugar- phosphate-sugar-phosphate… found in a polymer of nucleic acids. The relative strength of the backbone maintains the nucleotides in their specific sequence.
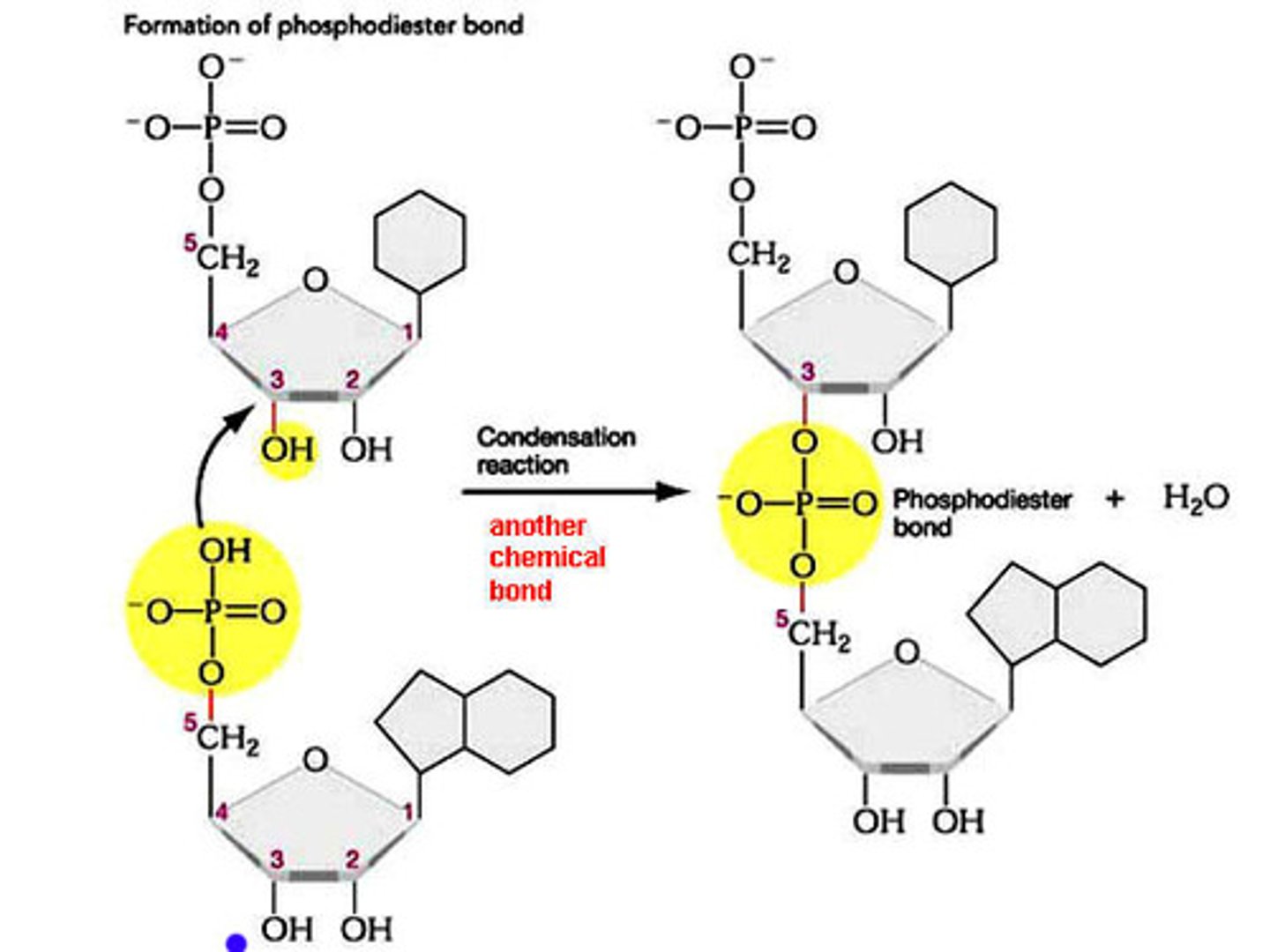
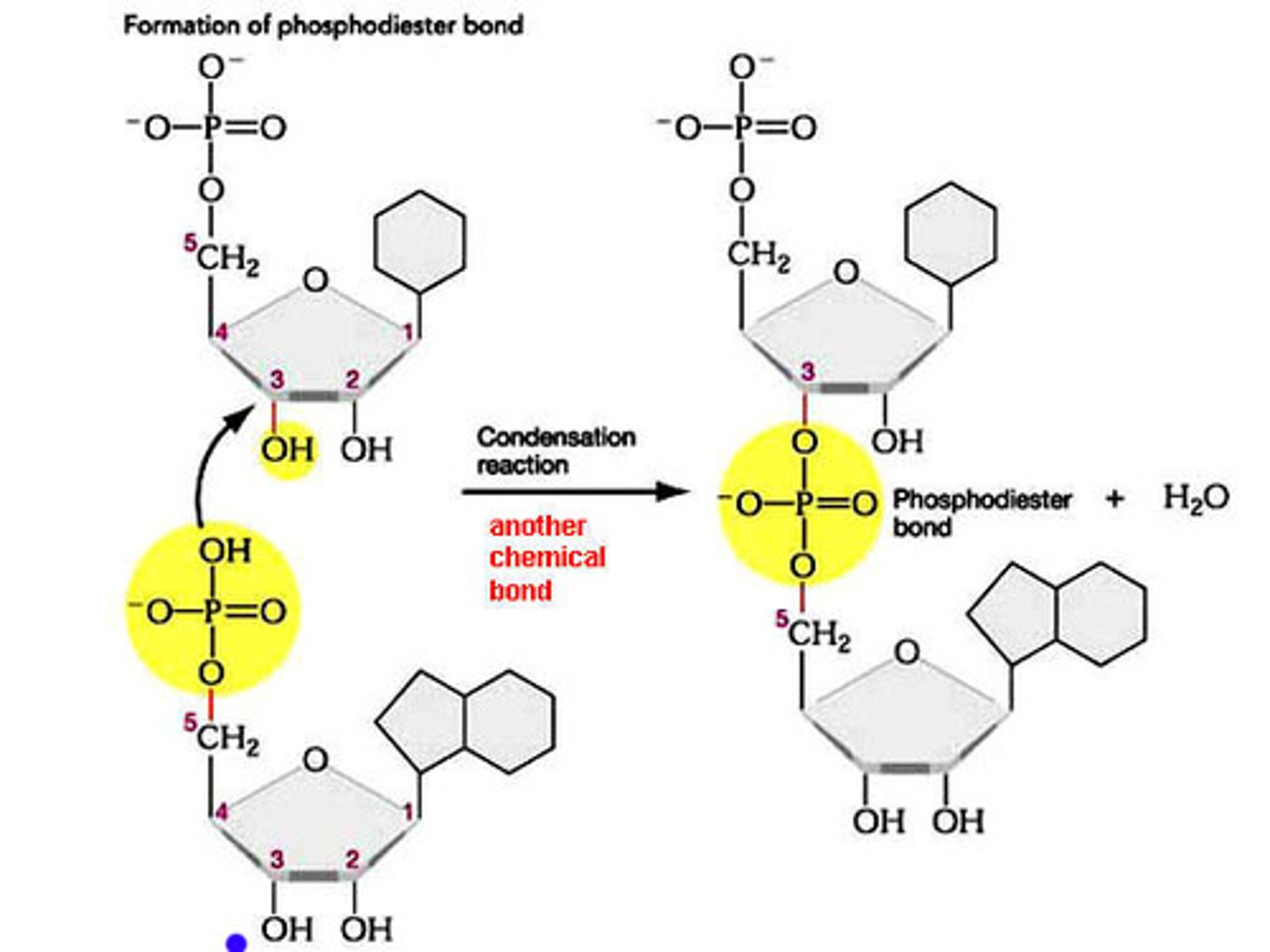
Explain how nucleotides can connect to form a nucleic acid polymer.
A1.2.3— Sugar–phosphate bonding and the sugar–phosphate “backbone” of DNA and RNA.
Nucleotides connect by creating covalent bonds between the sugar of one nucleotide and the phosphate group of another nucleotide in a condensation reaction.
The 5’ phosphate group on one nucleotide forms a new covalent bond with the 3' carbon on the pentose of the next nucleotide. Water is created as a biproduct.
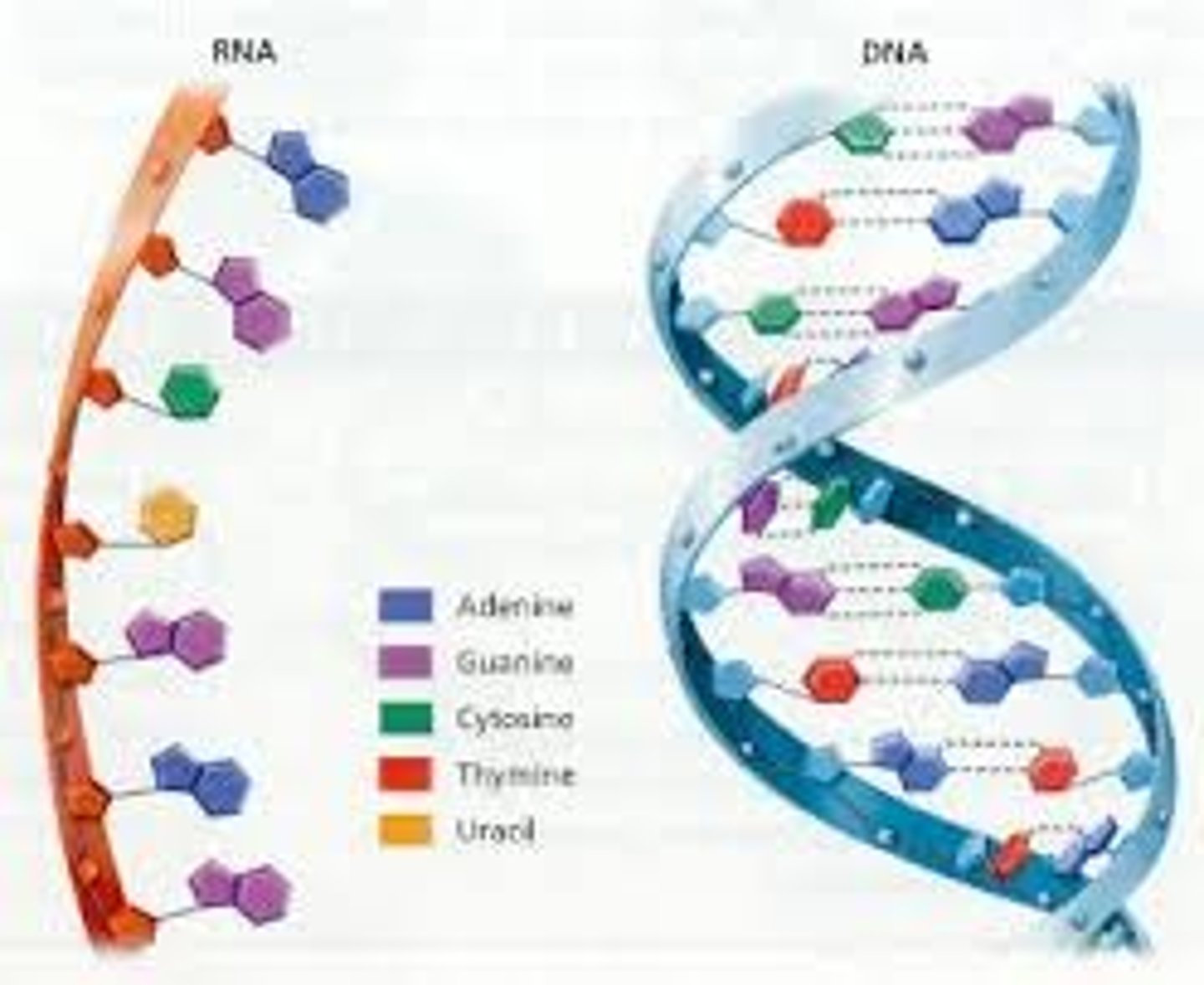
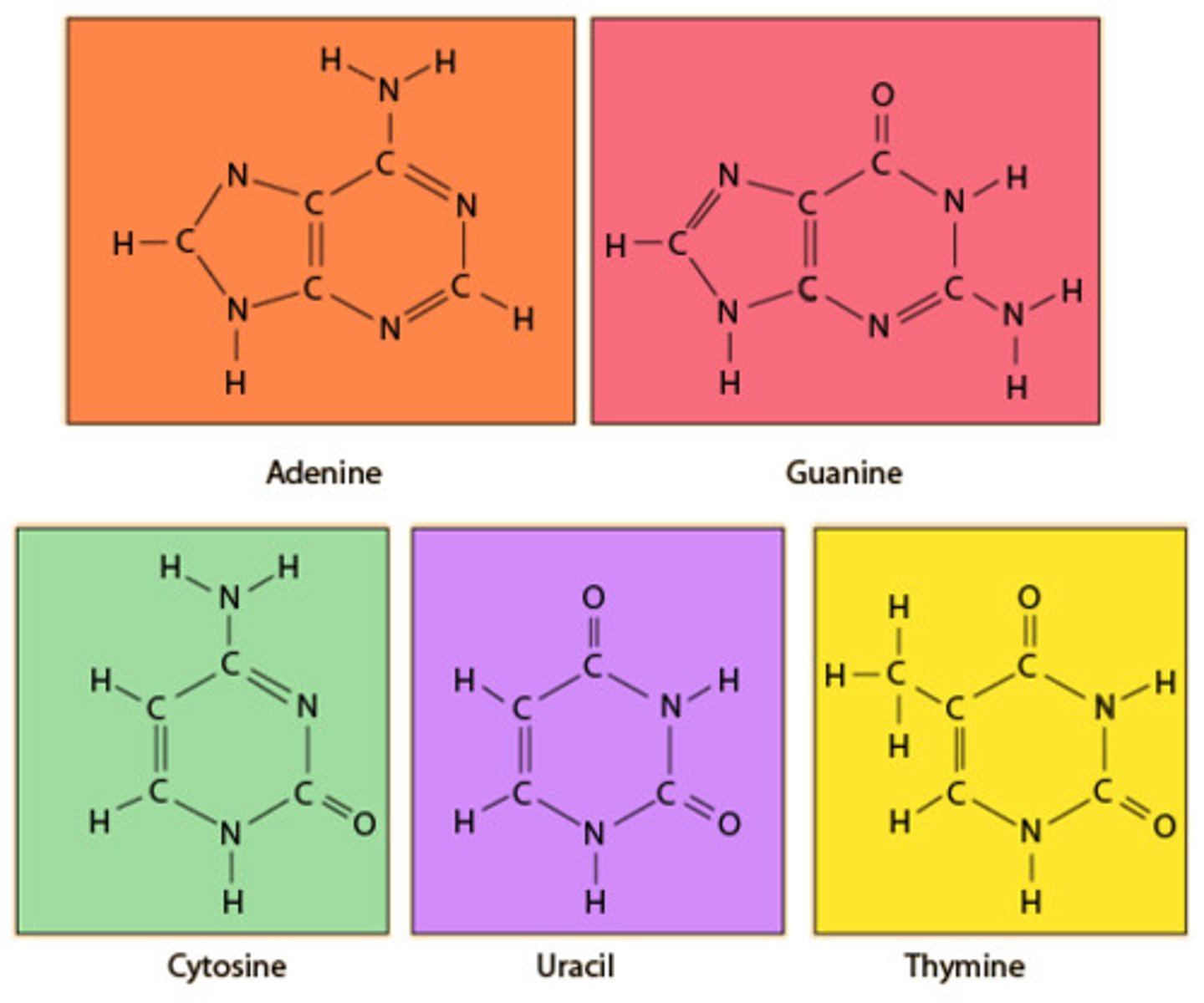
State the names of the nitrogenous bases found in DNA and RNA.
A1.2.4— Bases in each nucleic acid that form the basis of a code.
Cytosine (DNA and RNA)
Thymine (DNA only)
Guanine (DNA and RNA)
Adenine (DNA and RNA)
Uracil (RNA only)
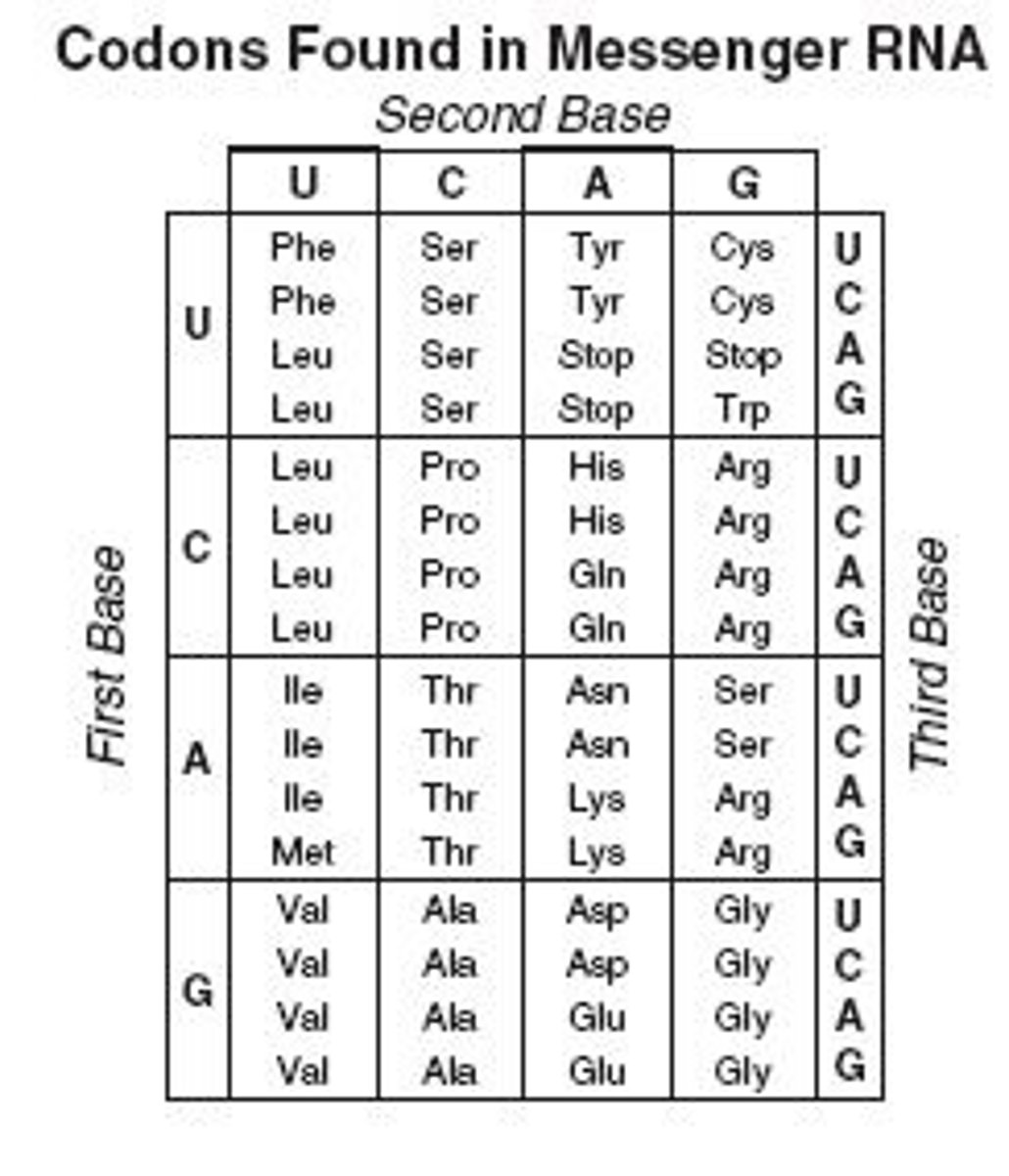
Outline how the sequence of bases in a nucleic acid serves as a ‘code.’
A1.2.4— Bases in each nucleic acid that form the basis of a code.
A code is a system in which one symbol signifies the meaning of another symbol. In the genetic code, a group of three nucleic acid bases signifies for an amino acid.A gene is a specific sequence of DNA nucleotides that codes for the making of a specific protein.
Define gene.
A1.2.4— Bases in each nucleic acid that form the basis of a code.
A gene is a specific sequence of nitrogenous bases in DNA nucleotides that codes for the making of a protein.
Describe the condensation reaction that forms a polymer of RNA from RNA nucleotides.
A1.2.5— RNA as a polymer formed by condensation of nucleotide monomers.
RNA nucleotides connect by creating covalent bonds between the ribose sugar of one nucleotide and the phosphate group of another nucleotide in a condensation reaction.
The 5’ phosphate group on one RNA nucleotide forms a new covalent bond with the 3' carbon on the ribose of the next nucleotide. Water is created as a biproduct.
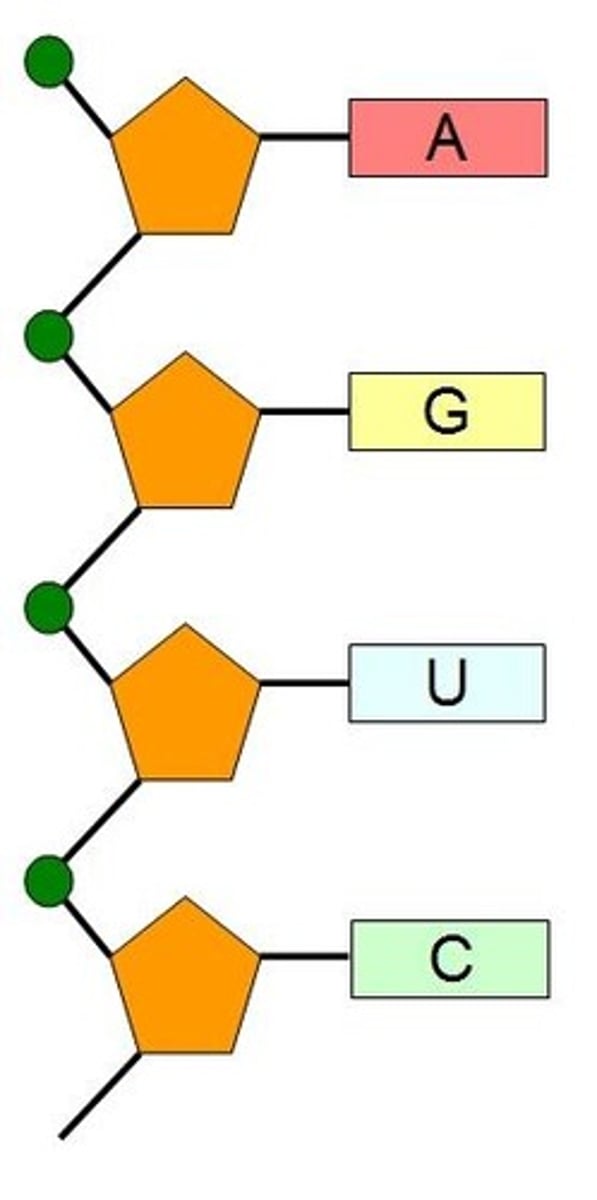
Draw a short section of an RNA polymer (using circle, pentagon and rectangle)
A1.2.5— RNA as a polymer formed by condensation of nucleotide monomers.
Include at least three RNA nucleotides, drawn as circle (phosphate), hexagon (ribose) and rectangle (base).
Be sure the phosphate of one nucleotide is connected to the 2'C of the adjacent nucleotide.
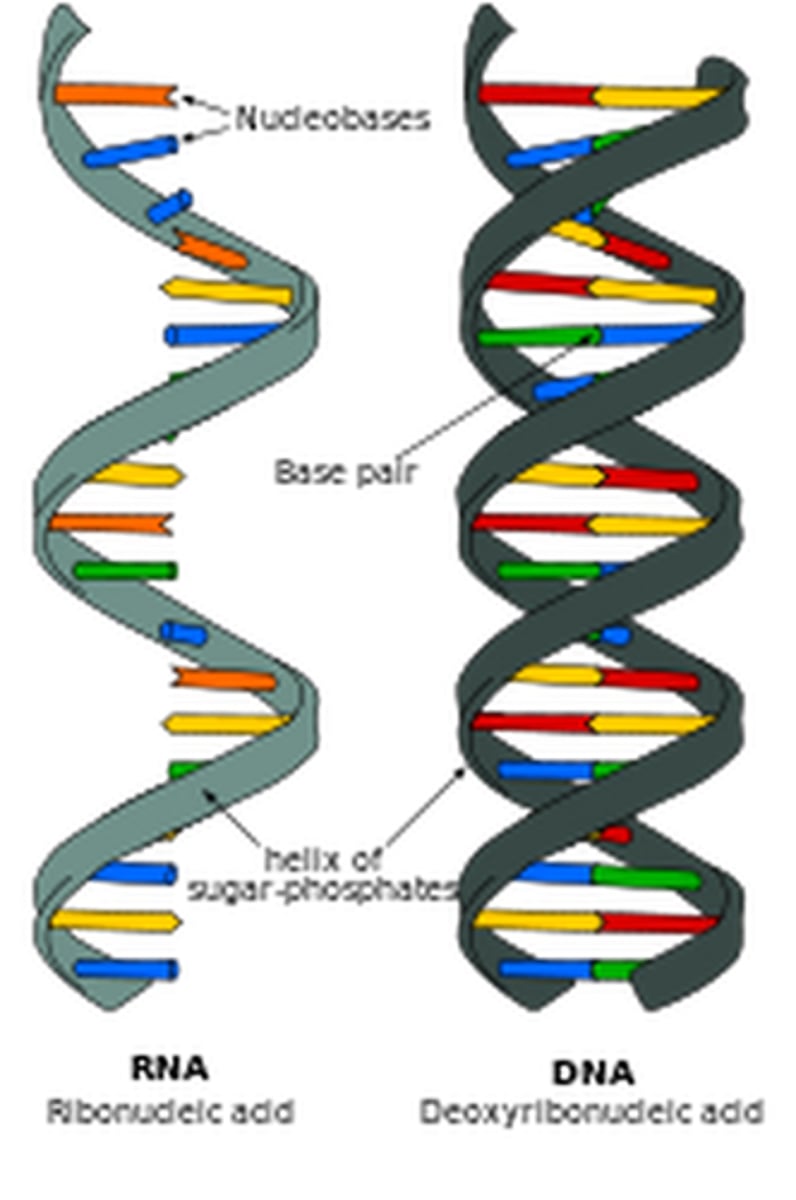
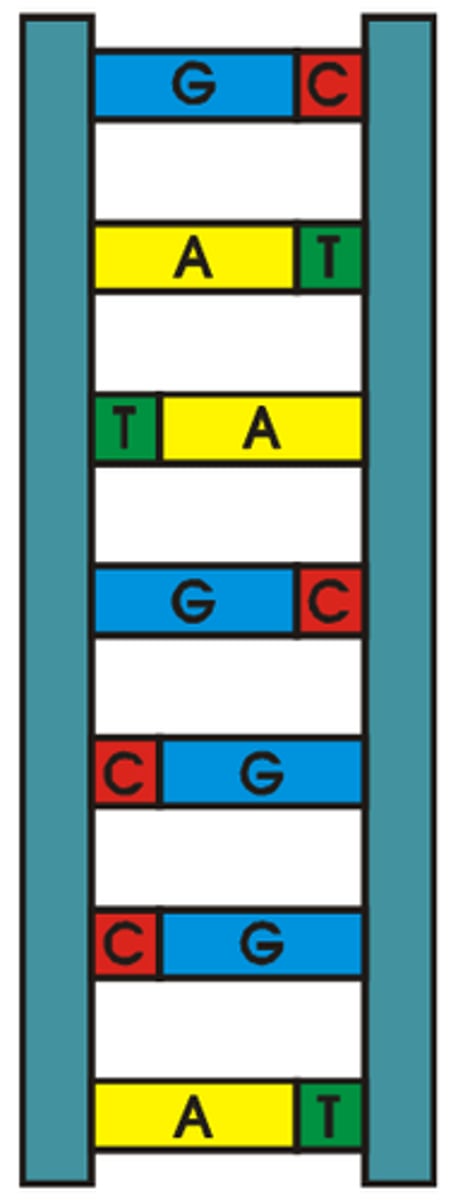
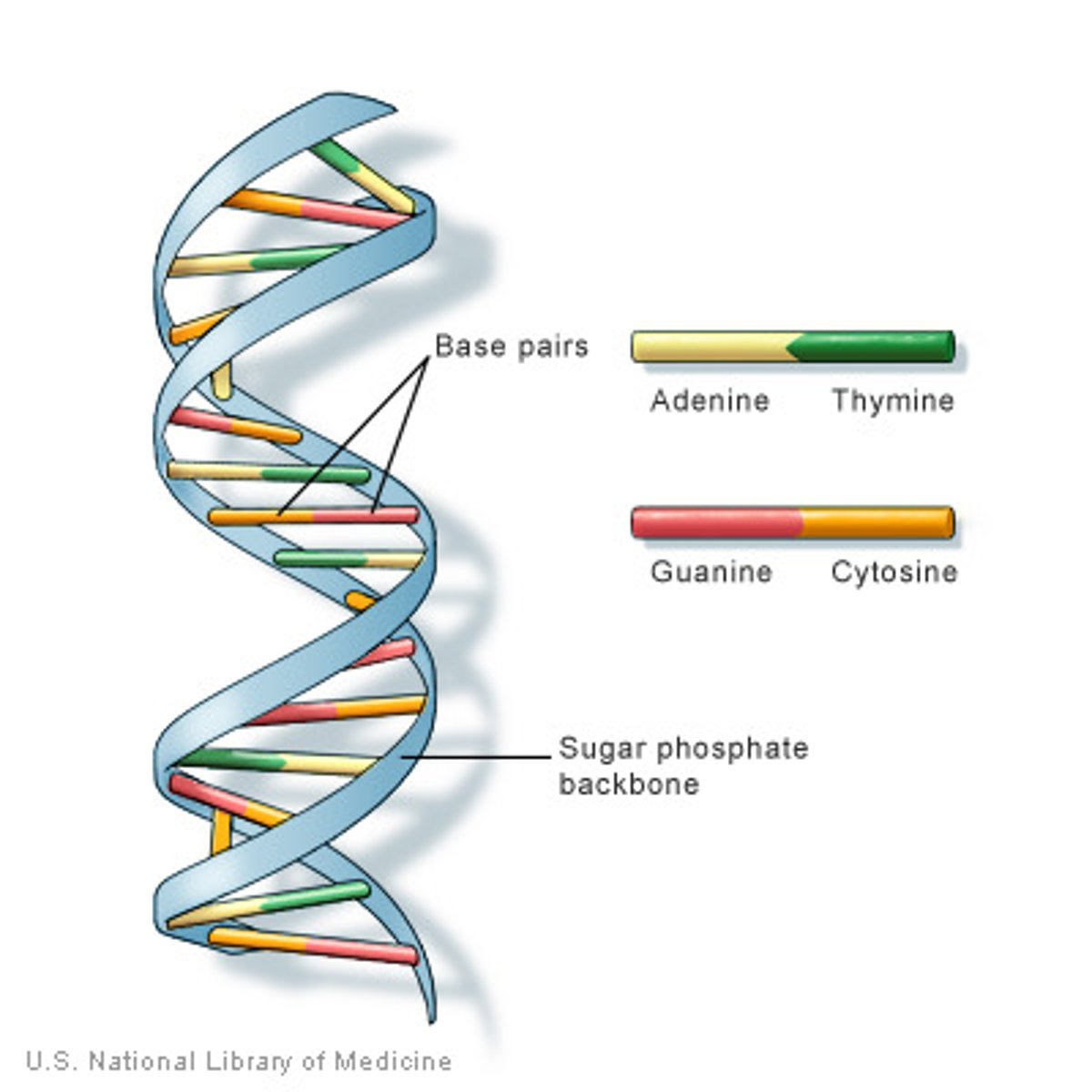
Describe the structure of a DNA double helix.
A1.2.6— DNA as a double helix made of two antiparallel strands of nucleotides with two strands linked by hydrogen bonding between complementary base pairs.
Two polymers of DNA nucleotides, each with a sugar-phosphate backbone, run in antiparallel directions. Complementary DNA nitrogenous bases (A-T, C-G) form hydrogen bonds between them, binding the two polymer stands ("double") so that they wind around each other ("helix")
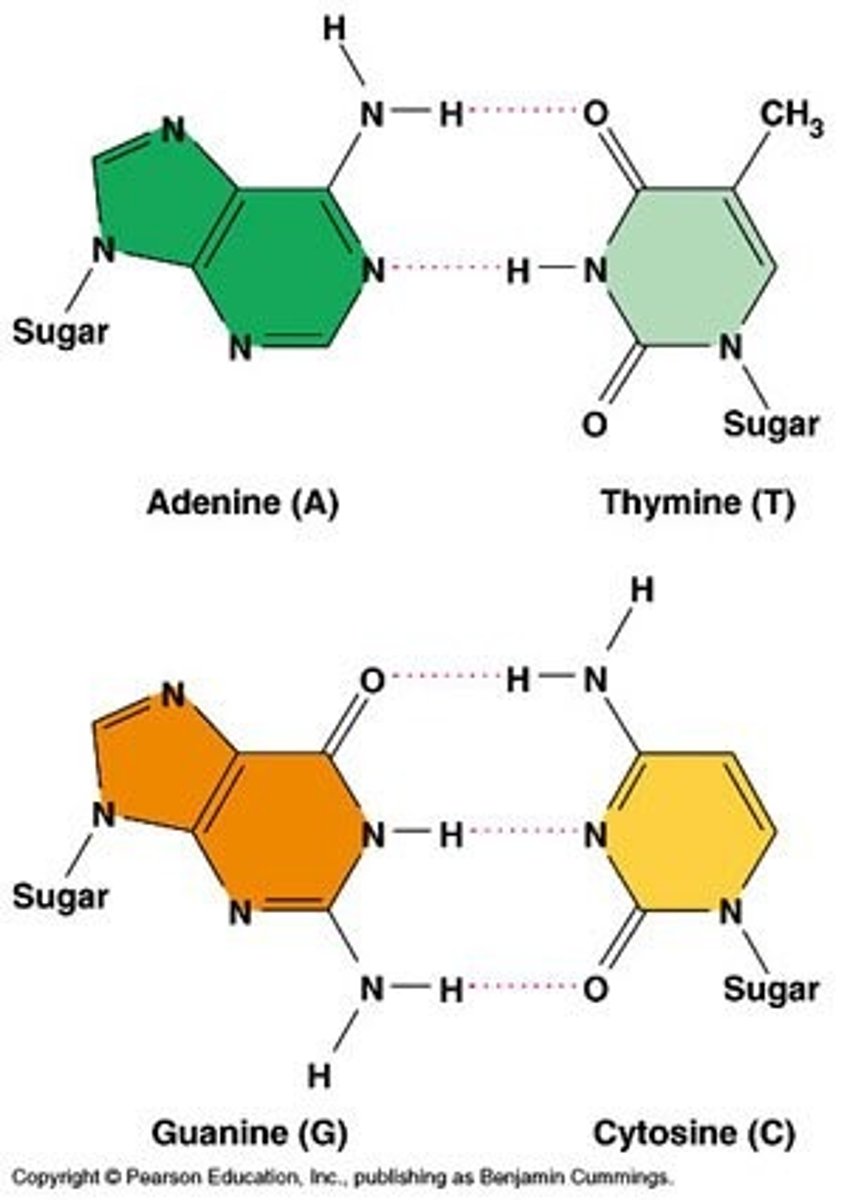
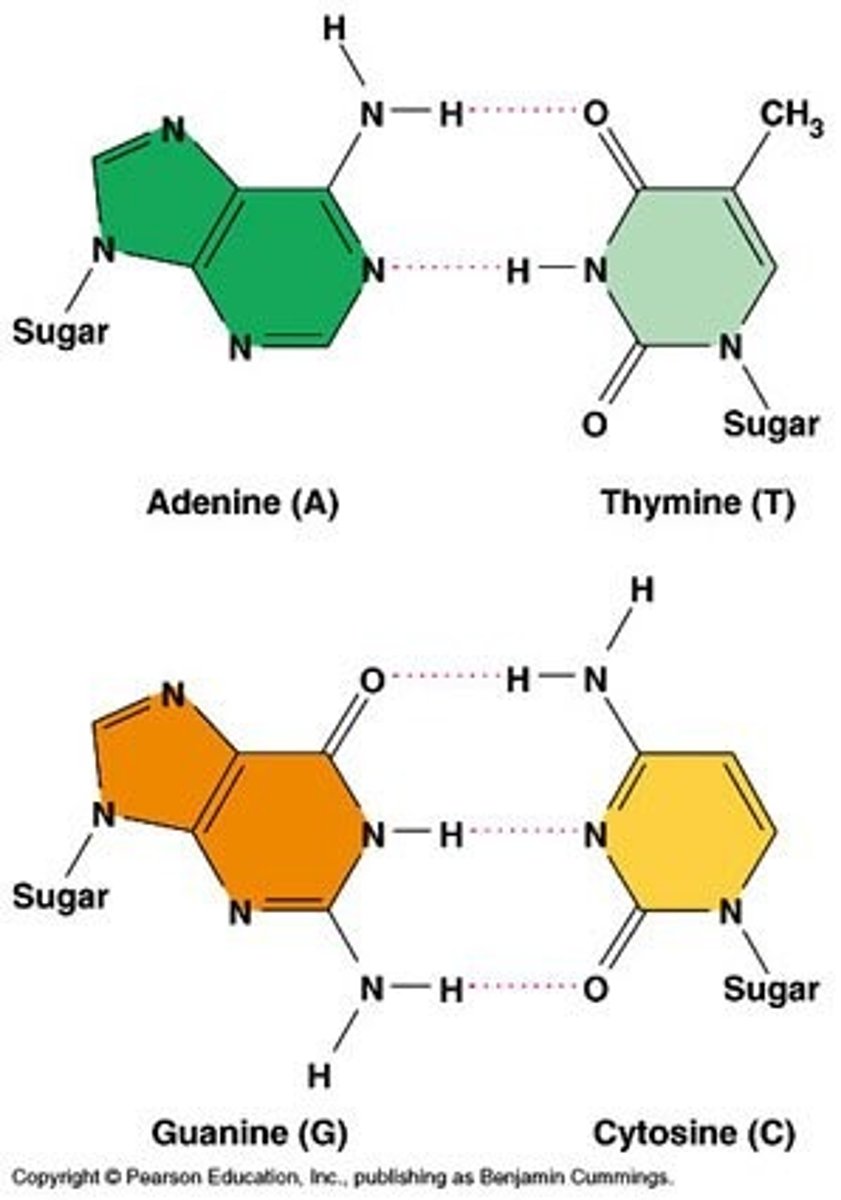
Outline the complementary base pairing rule, including the type and number of bonds between bases.
A1.2.6— DNA as a double helix made of two antiparallel strands of nucleotides with two strands linked by hydrogen bonding between complementary base pairs.
In DNA, the nitrogenous bases of two antiparallel strands form hydrogen bonds with each other. The complementary base pairing rule is that adenine only binds with thymine (with 2 H-bonds) and that guanine only binds with cytosine (with 3 H-bonds).
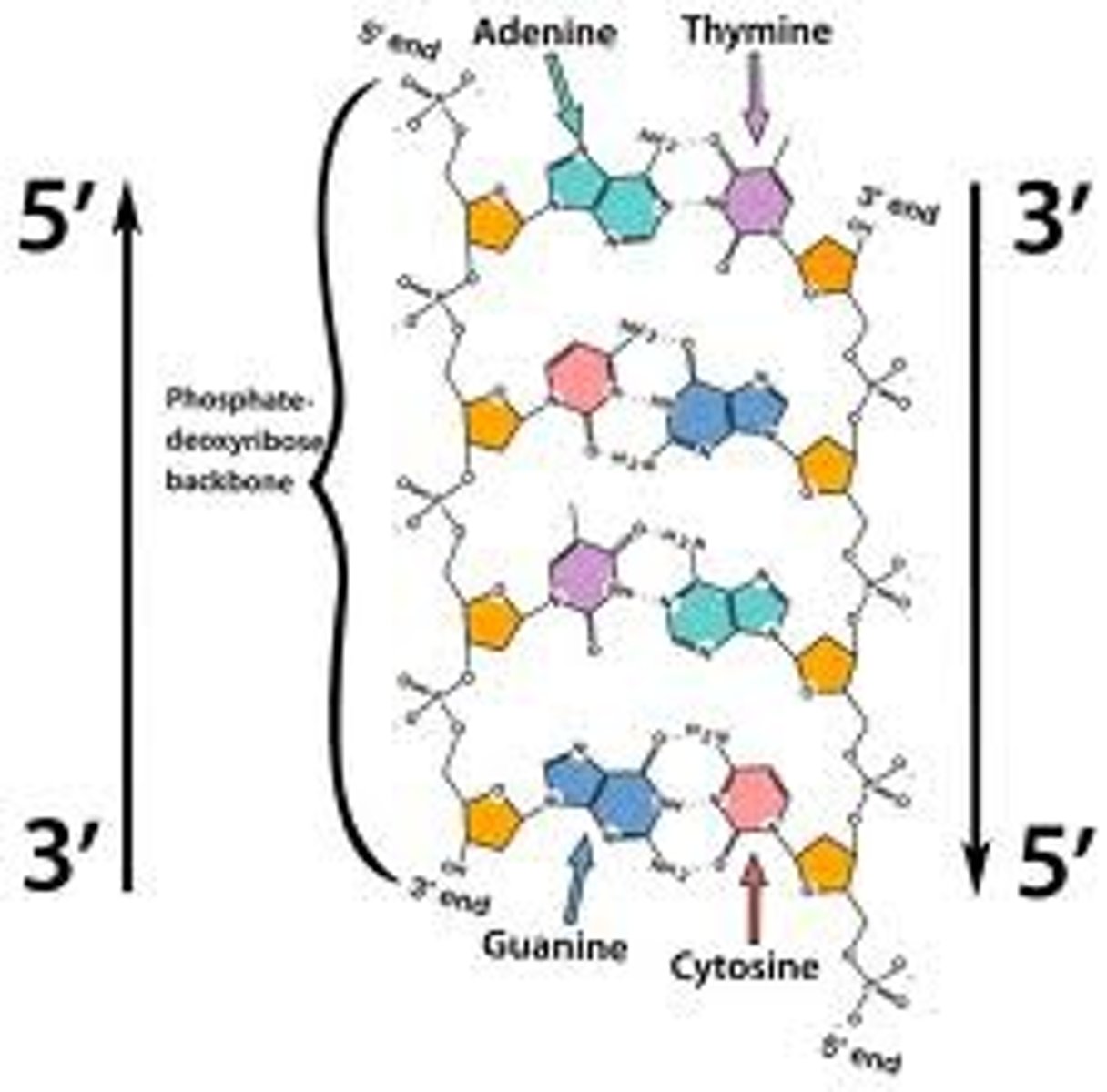
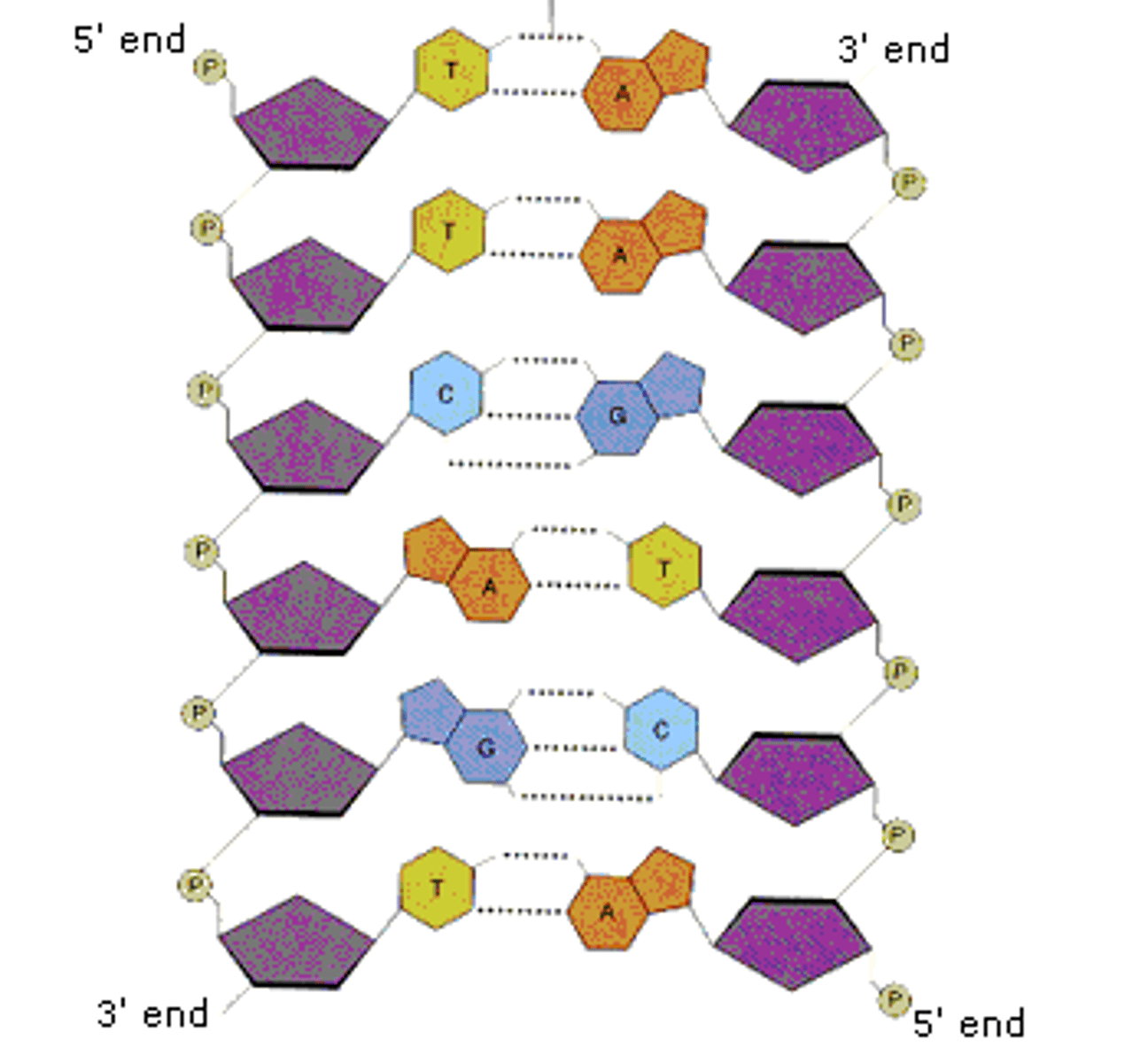
Define antiparallel in relation to DNA structure.
A1.2.6— DNA as a double helix made of two antiparallel strands of nucleotides with two strands linked by hydrogen bonding between complementary base pairs.
Adjacent molecules are oriented parallel to each other but oriented in opposite directions.
In DNA, one strand runs 5' to 3' and the complementary strand runs 3' to 5'
Compare and contrast the structure of DNA and RNA.
A1.2.7— Differences between DNA and RNA.
Both are nucleic acids formed through condensation of nucleotides. Both DNA and RNA have a sugar-phosphate backbone.
RNA
ribose
single stranded
nitrogenous bases A, G, C, U
Complementary paring A-U, C-G
DNA
deoxyribose
double stranded
nitrogenous bases A, G, C, T
Complementary pairing A-T, C-G
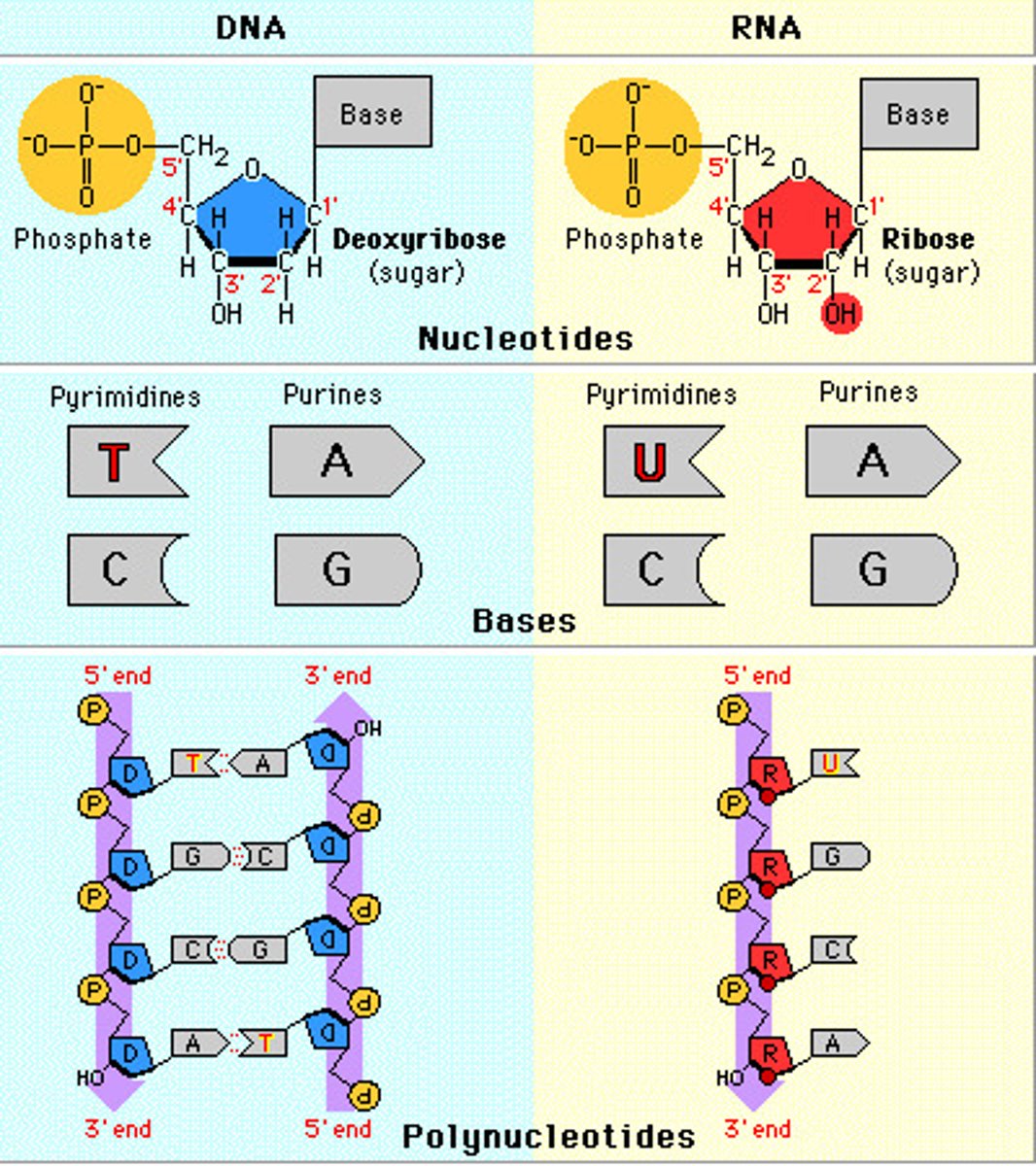
Compare and contrast the functions of DNA and RNA.
A1.2.7— Differences between DNA and RNA.
DNA:
Passes heredity information between generations of cells
Codes for making RNA during transcription
RNA:
Codes for making proteins during translation
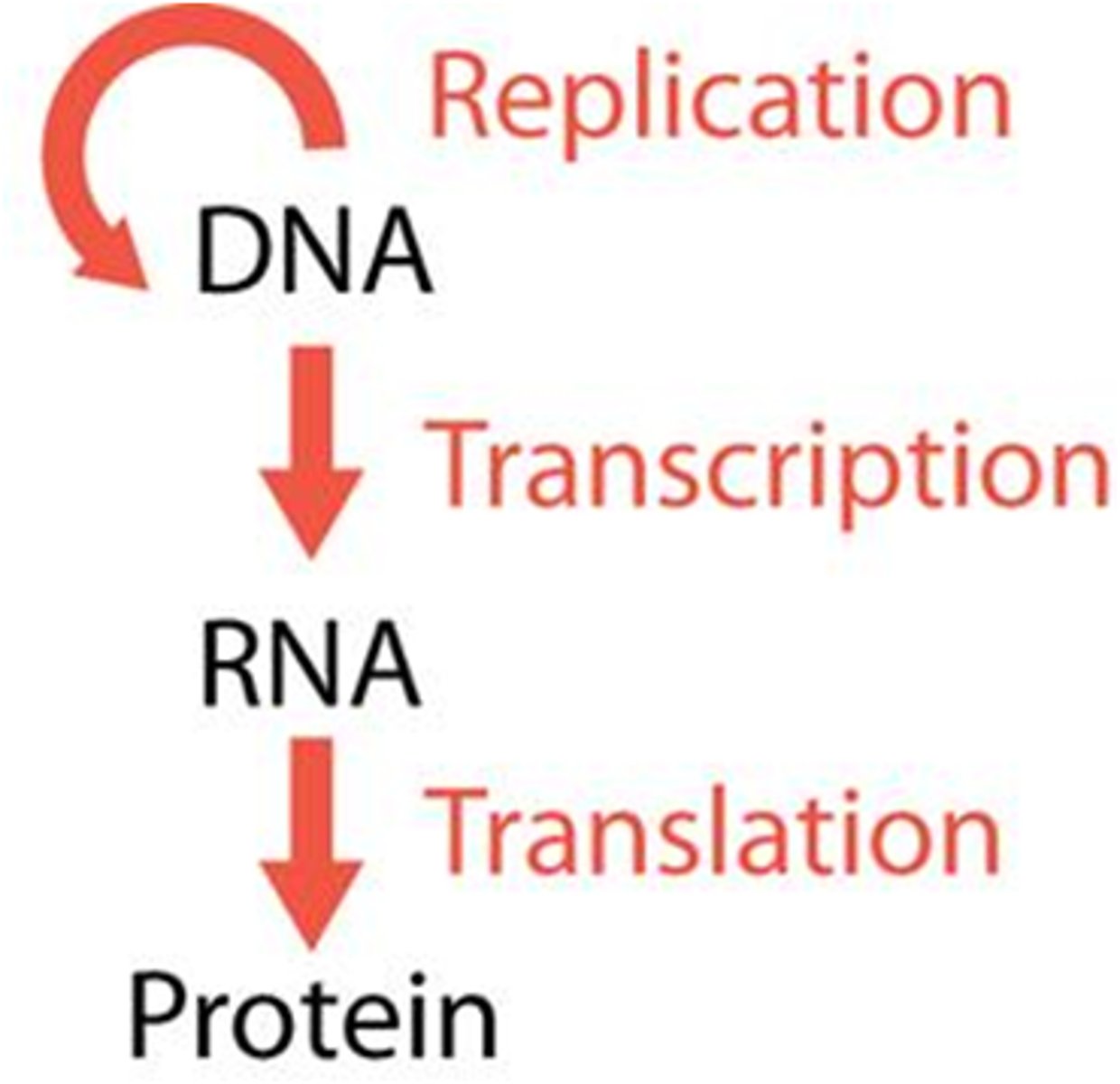
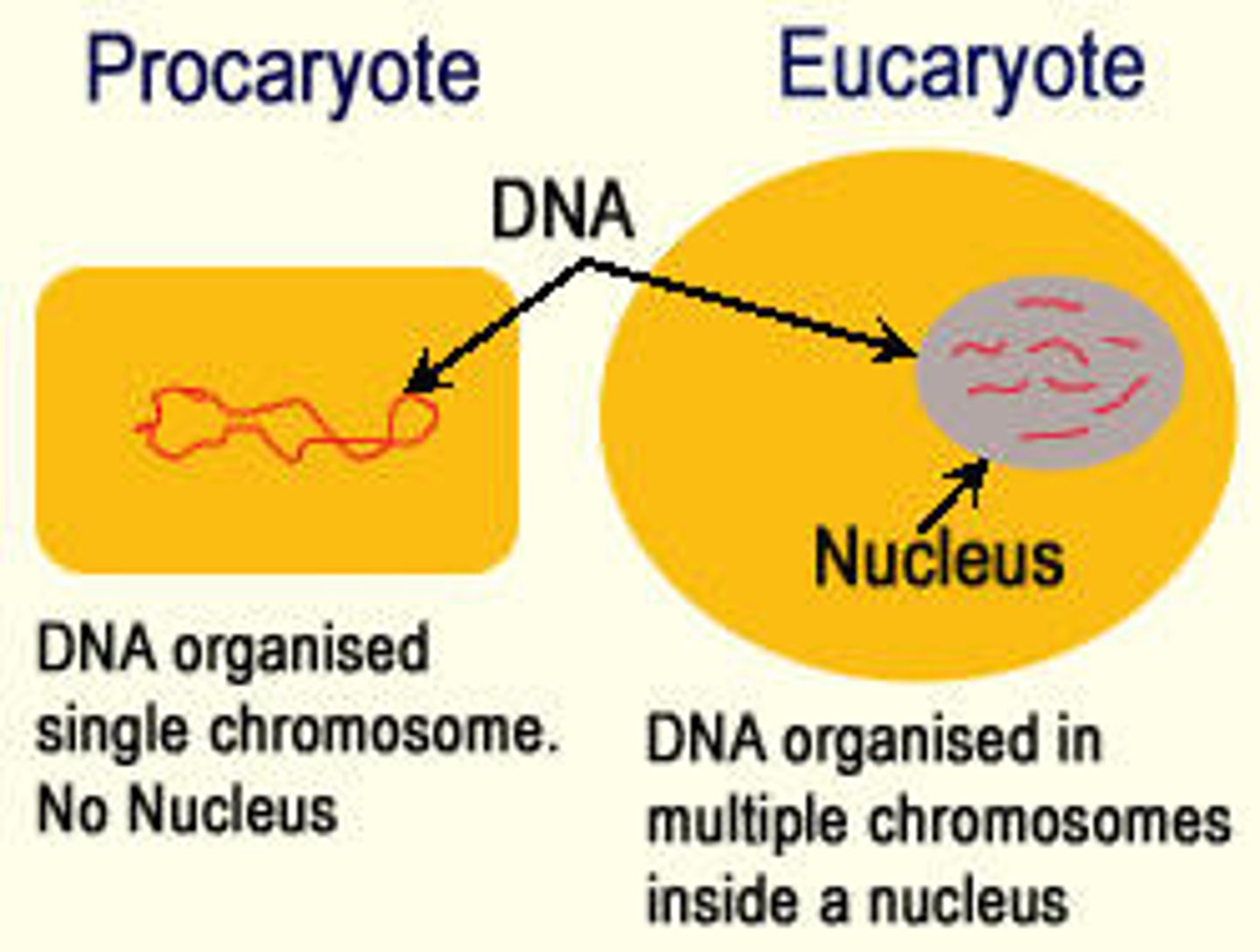
Compare and contrast the location of DNA and RNA in prokaryotic and eukaryotic cells.
A1.2.7— Differences between DNA and RNA.
Eukaryotic Cells
Both DNA and RNA are found in the nucleus.
DNA also in mitochondria and chloroplasts.
RNA also in cytoplasm and as part of ribosomes (free or bound to rough ER)
Prokaryotic Cells
Both DNA and RNA are in the cytoplasm.
DNA is clumped in a region called the nucleoid.
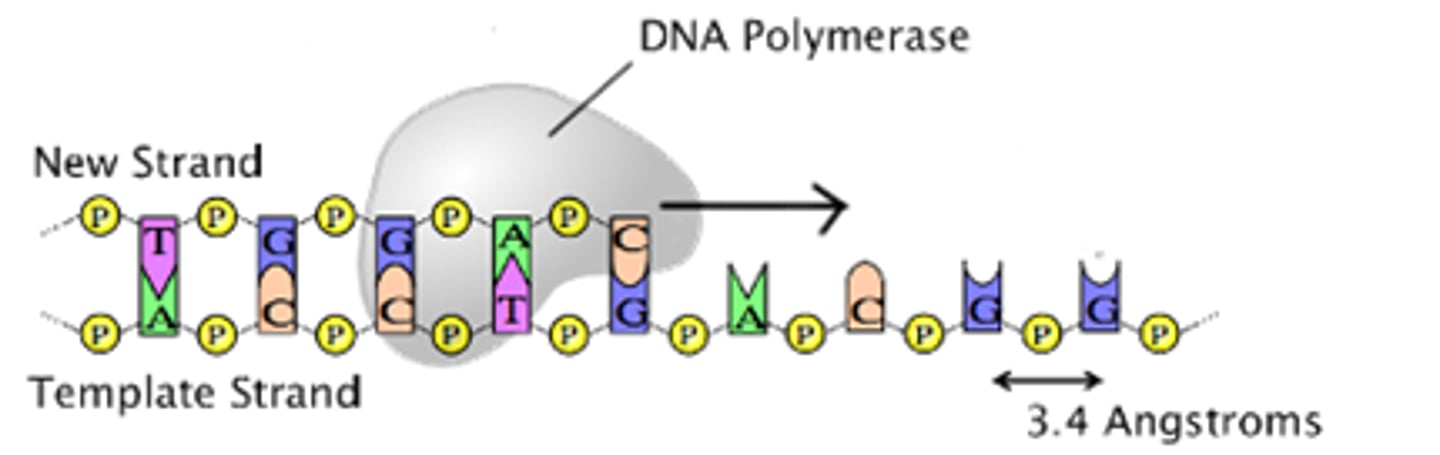
Explain the role of complementary base pairing in maintaining the DNA sequence during DNA replication.
A1.2.8— Role of complementary base pairing in allowing genetic information to be replicated and expressed.
During DNA replication, the two strands of a "parent" DNA molecule are broken apart. Each of these strands serves as a template for the creation of a new "daughter" strand. Because of the base pairing rule, the parent template strand will always code for the complementary sequence of nucleotides in the daughter strand (A to T, C to G). The complementary base paring will maintain the sequence of the DNA from generation to generation.
Outline the role of complementary base pairing in transmitting the genetic code in transcription.
A1.2.8— Role of complementary base pairing in allowing genetic information to be replicated and expressed.
During transcription, one of the the two strands of a DNA molecule is used as a template for the creation of an RNA strand. Because of the base pairing rule, the DNA template strand will always code for the complementary sequence of RNA nucleotides in the (A to U, C to G). The complementary base paring will maintain the sequence of the gene as mRNA is translated into protein.
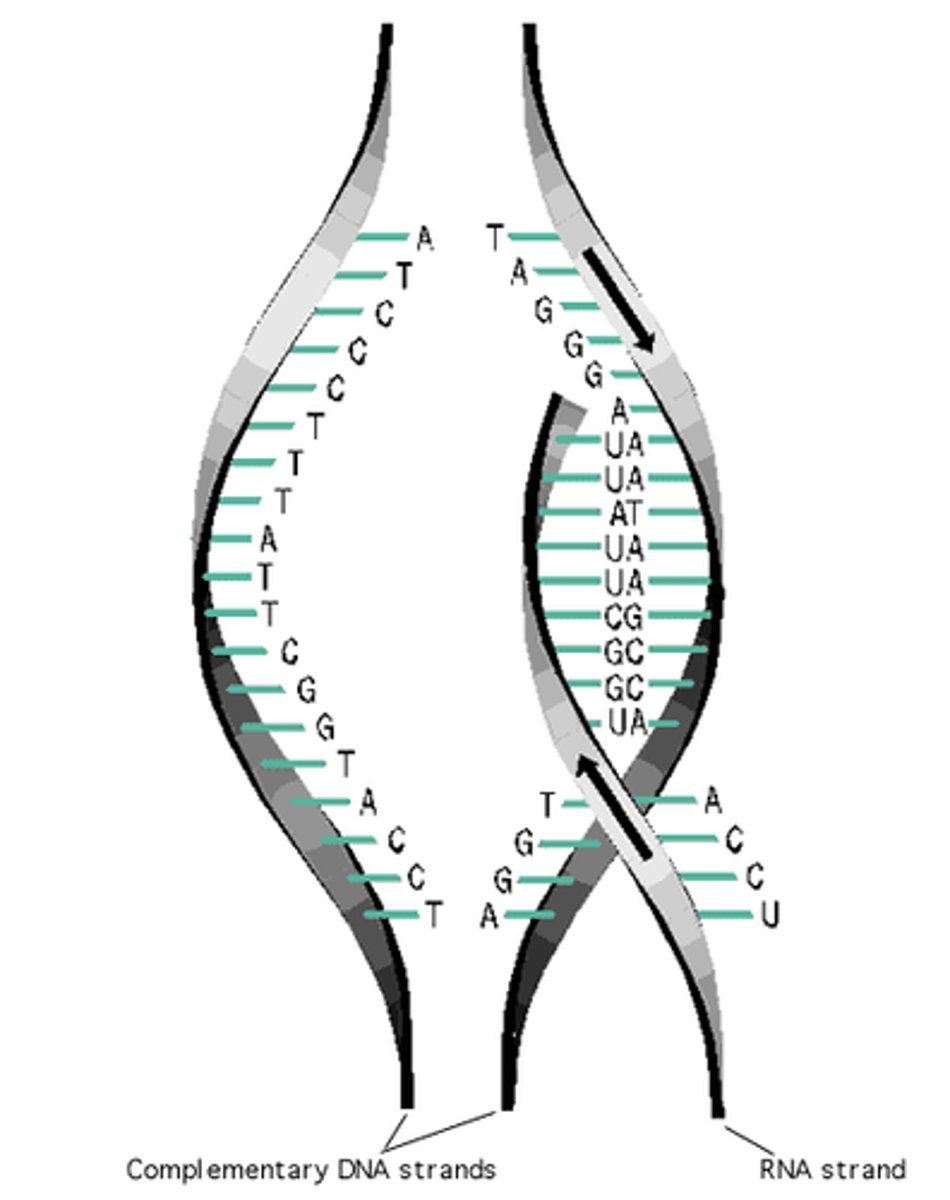
Outline the role of complementary base pairing in transmitting the genetic code in translation.
A1.2.8— Role of complementary base pairing in allowing genetic information to be replicated and expressed.
During translation, an RNA strand is used as a template for the creation of a polypeptide. Because of the base pairing rule, the mRNA codon will only bind with the complementary tRNA anticodon (A to U, C to G). The complementary base paring ensures the correct amino acid are brought in the correct sequence to the ribosome.
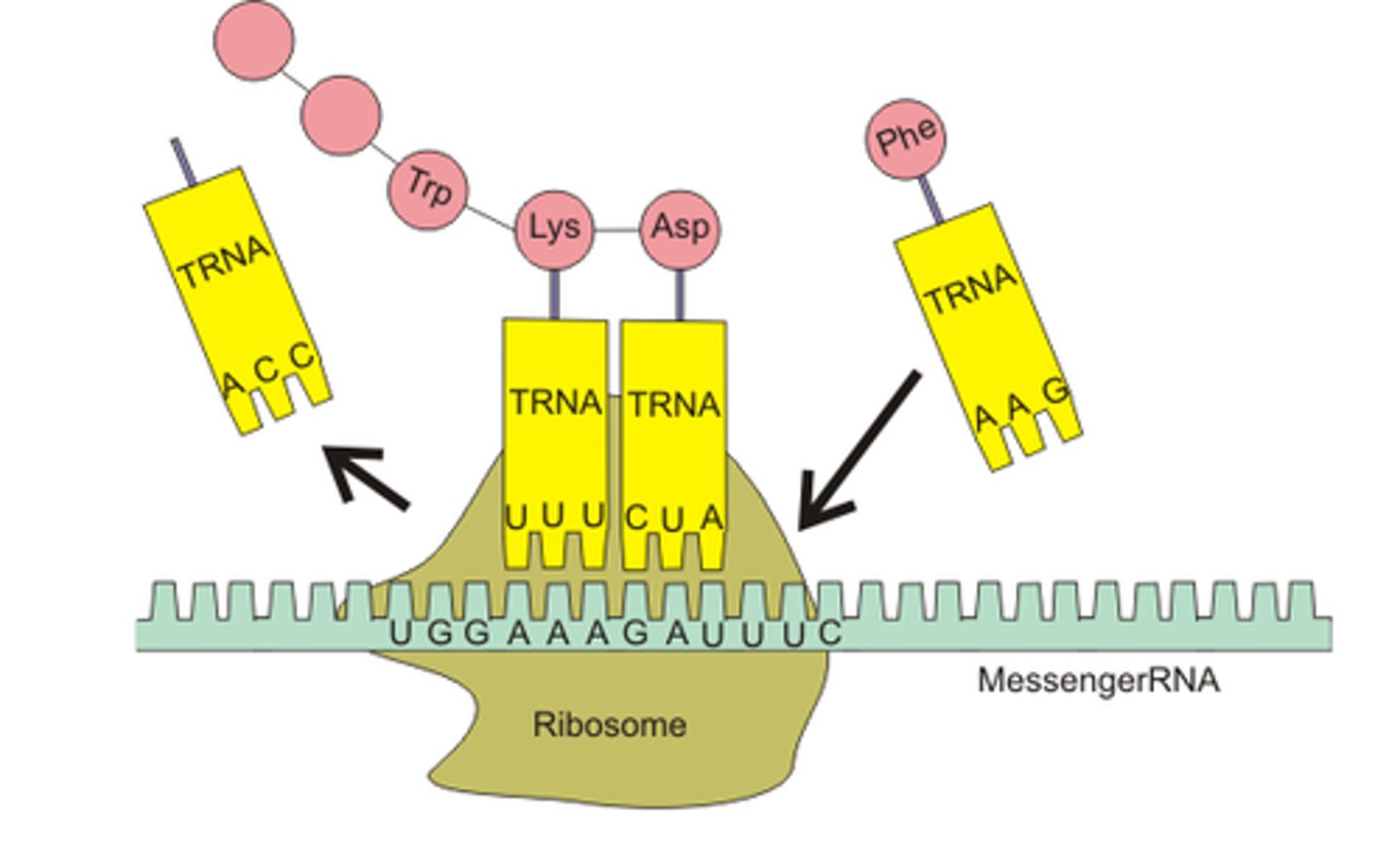
Outline why there is a limitless diversity of DNA base sequences.
A1.2.9— Diversity of possible DNA base sequences and the limitless capacity of DNA for storing Information.
There are four nitrogenous bases in DNA (A, T, C and G). These 4 bases are components of nucleotides that can form a DNA molecule in any order and of any length.
Define universal in relation to the genetic code.
A1.2.10— Conservation of the genetic code across all life forms as evidence of universal common ancestry.
Universal means that the characteristic is shared by all life. A universal generic code means that all life uses essentially the same code when translating information stored in genes into a polypeptide.
Outline why conservation of the genetic code across all forms of life is evidence of common ancestry.
A1.2.10— Conservation of the genetic code across all life forms as evidence of universal common ancestry.
Using inductive reasoning, it can be concluded that the use of the same genetic code across all forms of life indicates that all organisms inherited the use of the code from a common ancestor. The alternative, that all forms of life independently developed use of the same genetic code, is an illogical hypothesis.
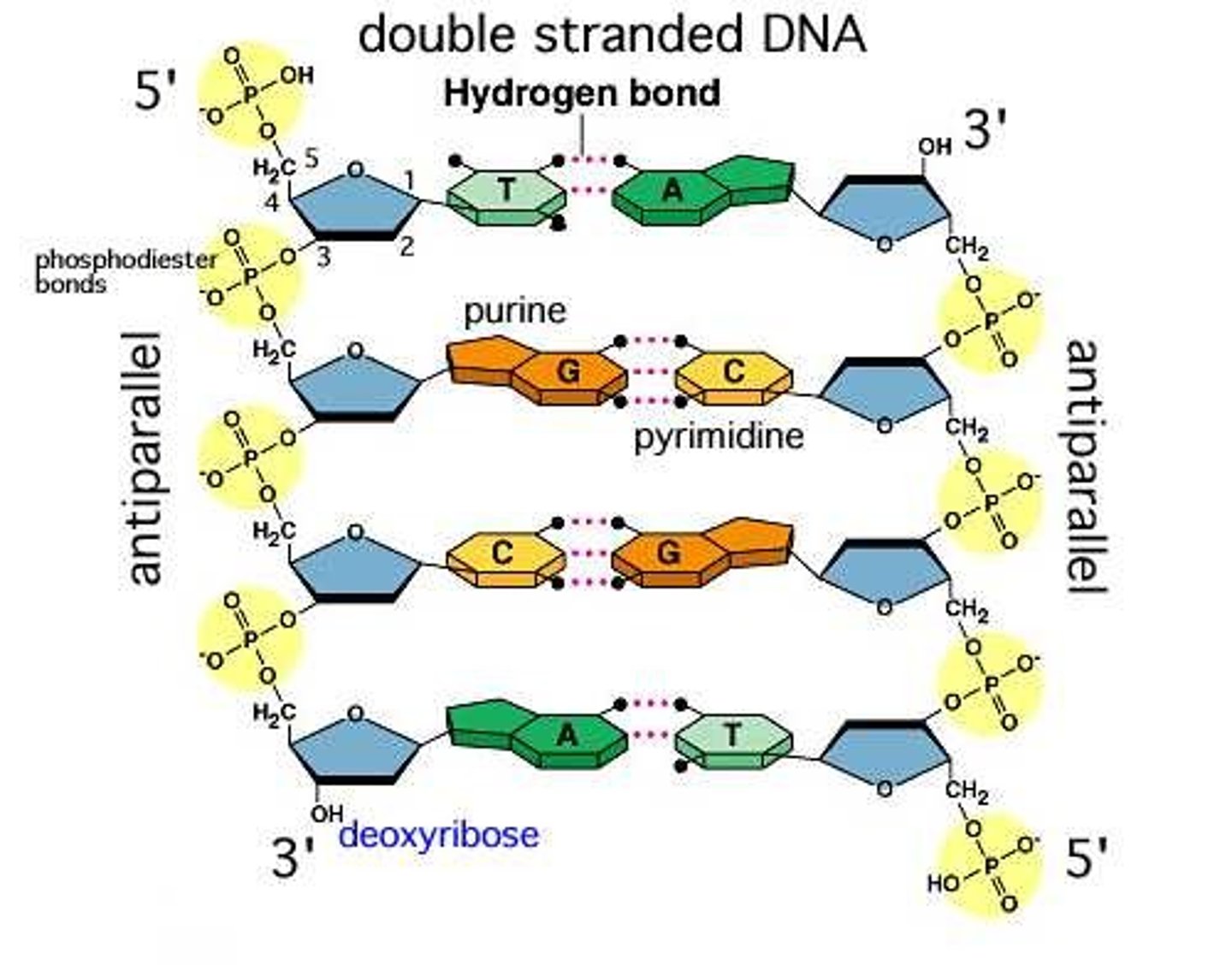
Identify and label the 5’ and 3’ ends on a DNA or RNA diagram.
AHL A1.2.11— Directionality of RNA and DNA.
Each end of DNA nucleotide has a number. One end is referred to as 5' (five prime) and the other end is referred to as 3' (three prime). The 5' and 3' designations refer to the number of the carbon atom in a deoxyribose sugar molecule. The 5' end is identified by the presence of the phosphate group and the 3' end is identified as ending in the pentose sugar (ribose or deoxyribose).
In DNA, one strand will run from 5' to 3' and the complementary strand will run anti-parallel, from 3' to 5'.
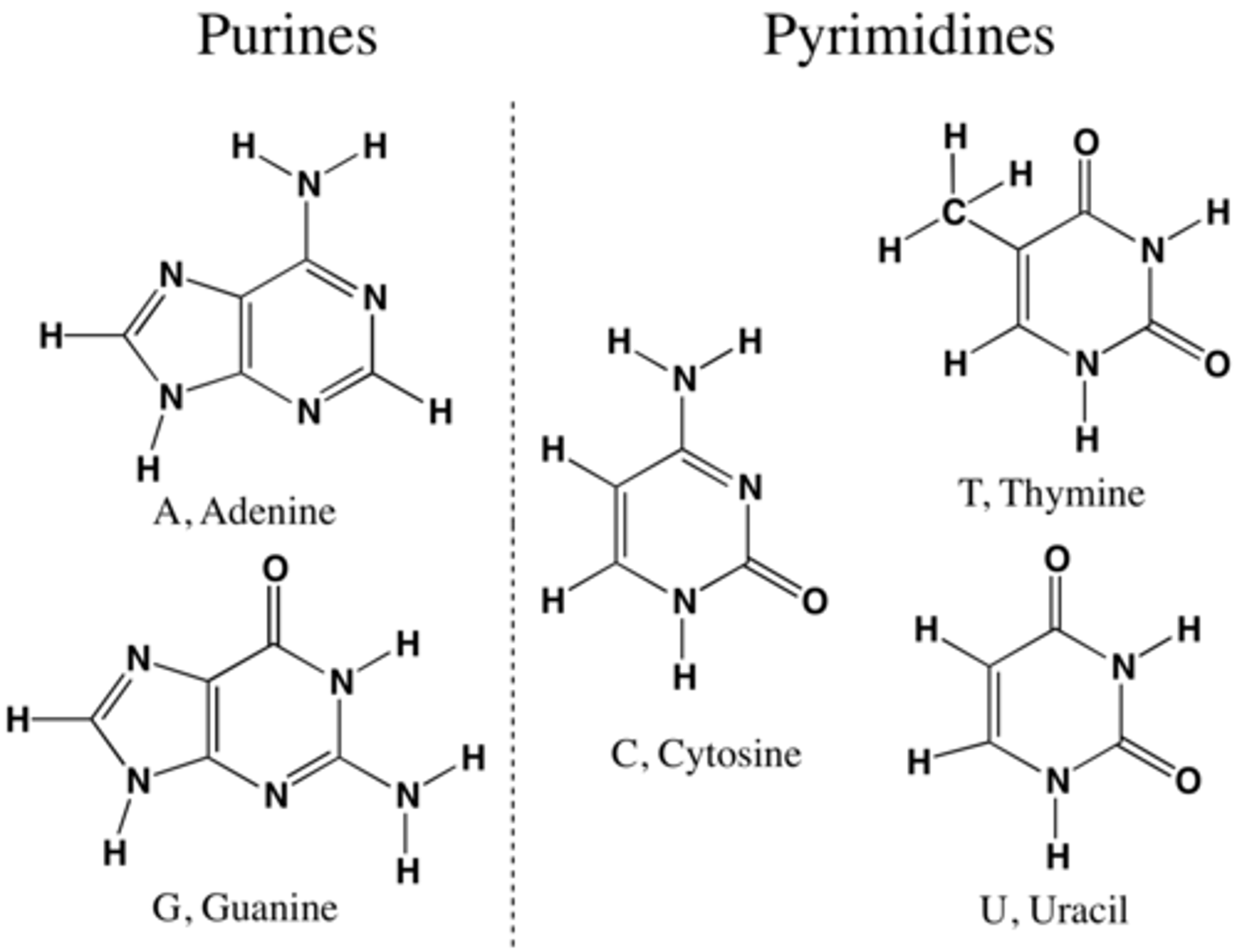
Compare and contrast the structures of purines and pyrimidines.
AHL A1.2.12— Purine-to-pyrimidine bonding as a component of DNA helix stability.
Both purines and pyrimidines are nitrogenous bases of DNA and RNA.
Pyrimidine: single ring nitrogenous bases
Cytosine
Thymine
Uracil
Purine: double ring nitrogenous bases
Guanine
Adenine
State that in DNA, a purine forms hydrogen bonds with a pyrimidine.
AHL A1.2.12— Purine-to-pyrimidine bonding as a component of DNA helix stability.
In DNA, a purine complementary base pairs to a pyrimidine using hydrogen bonds
In DNA and RNA, guanine bonds with cytosine with three hydrogen bonds.
In DNA, adenine bonds with thymine with two hydrogen bonds.
In RNA, adenine bonds with uracil with two hydrogen bonds
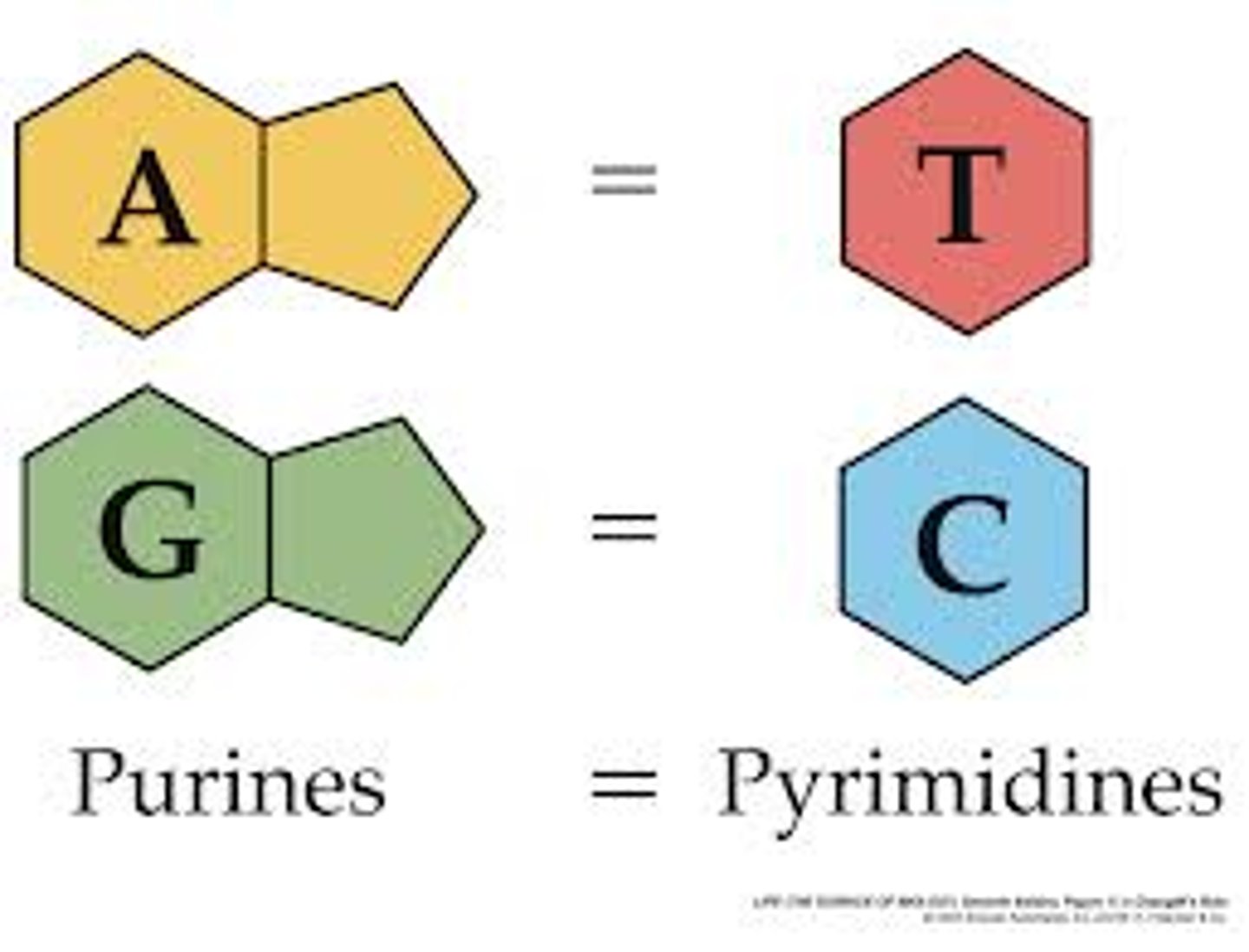
Given a diagram of DNA, identify the four bases of DNA based on purine or pyrimidine and the number of hydrogen bonds.
AHL A1.2.12— Purine-to-pyrimidine bonding as a component of DNA helix stability.
Purines have two rings. If it can form 2 H-bonds it is adenine and if it can form 3 H-bonds it is guanine.
Pyrimidines have one ring. If it can form 2 H-bonds it is thymine and if it can form 3 H-bonds it is cytosine.
Describe the structure of eukaryotic DNA and associated histone proteins during interphase (chromatin).
AHL A1.2.13— Structure of a nucleosome.
To compact DNA while regulating gene accessibility for transcription, eukaryotic organisms organize their genomes:
1. DNA double helix.
2. DNA wraps around histone proteins, forming nucleosomes and the "beads on a string" structure.
3. Multiple nucleosomes wrap into a fibre (chromatin).
4. Supercoiling of the chromatin produces the chromosome (during mitosis and meiosis). Supercoiling refers to the repeated twisting and winding of the DNA strand. Supercoiling functions to reduce the space required for DNA packaging, allowing for more compact storage of DNA.
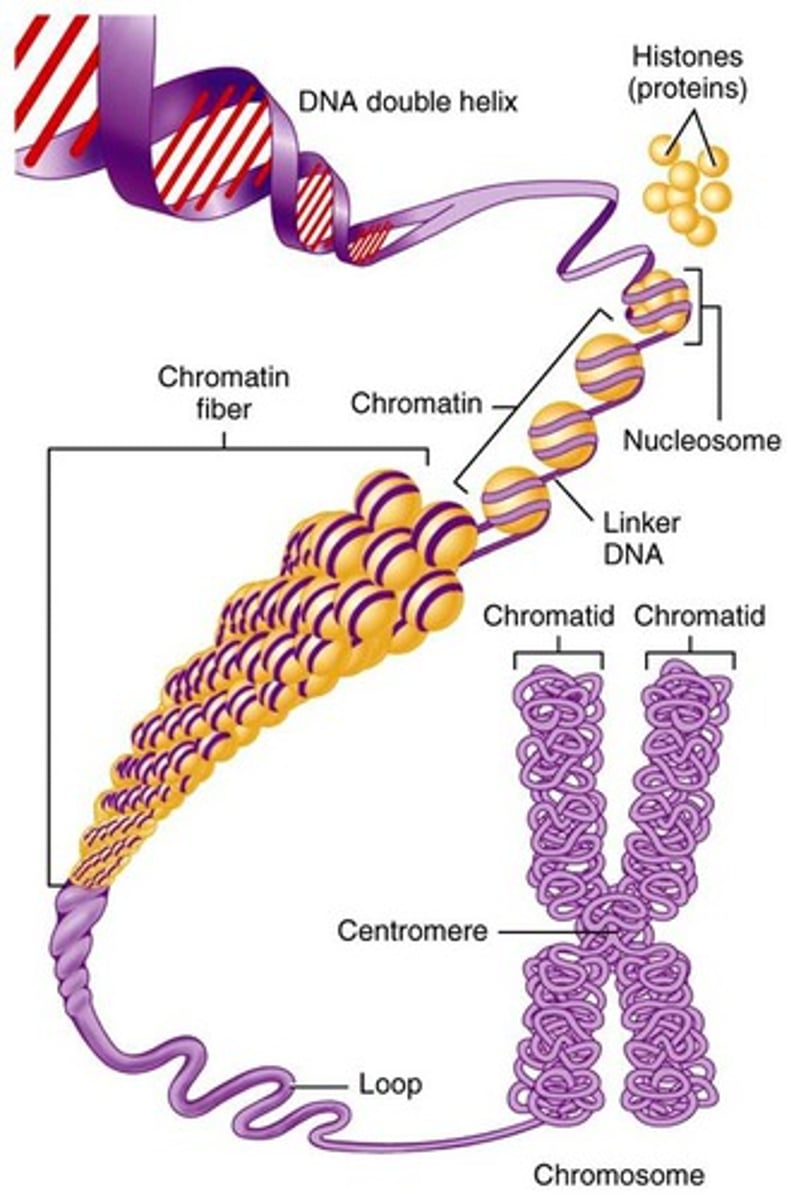
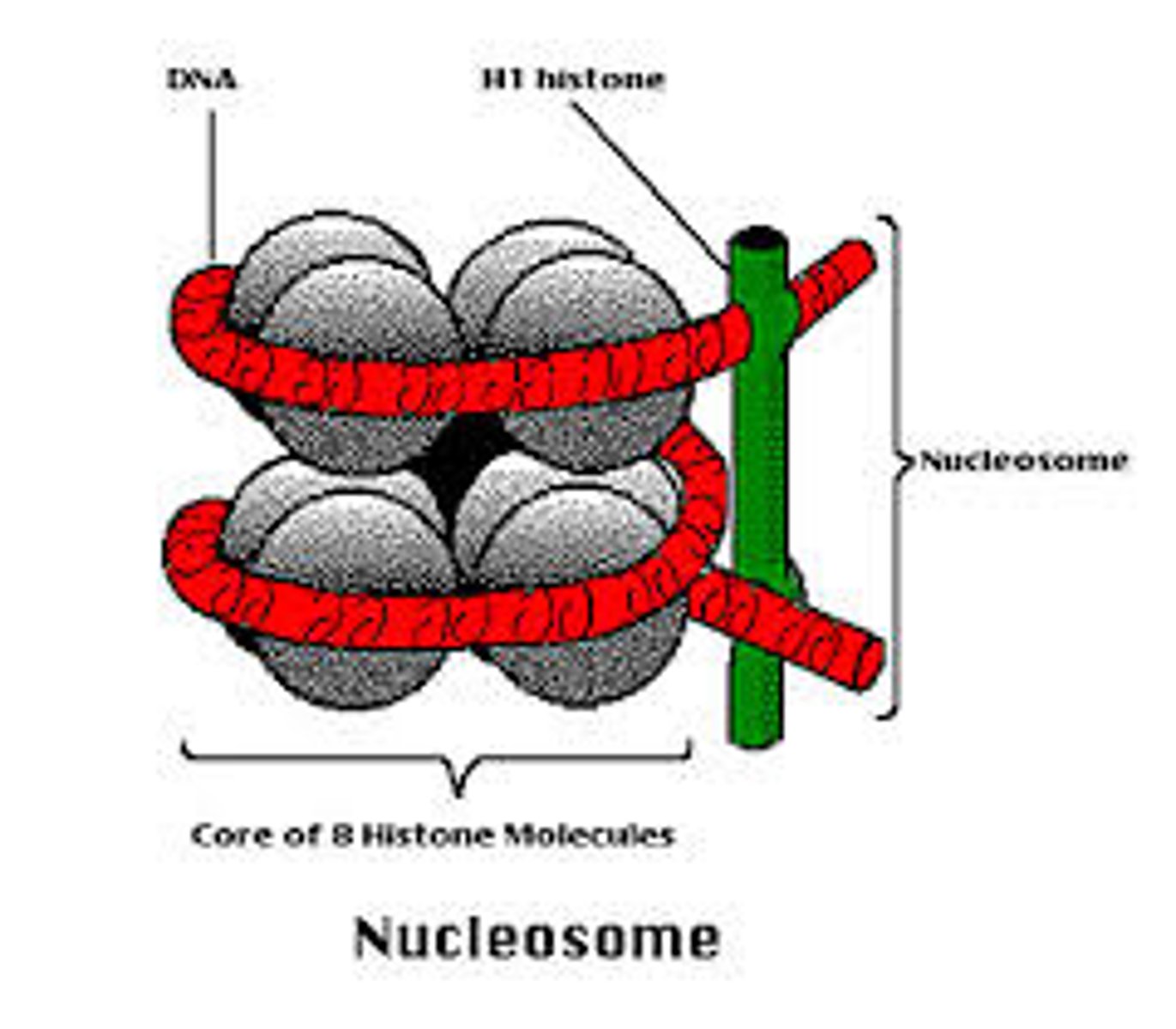
Draw and label the structure of a nucleosome, including the H1 protein, the octamer core proteins, linker DNA and two wraps of DNA.
AHL A1.2.13— Structure of a nucleosome.
The nucleosome is the basic unit of DNA packaging in eukaryotes. Each nucleosome is composed of two turns of DNA wrapped around a group of eight histone proteins (called an octamer core). Each nucleosome connects to the adjacent nucleosomes through another type of histone protein (called the H1) and a region of "linker" DNA.
State the experimental question being tested in the Hershey and Chase experiment.
AHL A1.2.14— Evidence from the Hershey–Chase experiment for DNA as the genetic material.
Both protein and nucleic acids are part of the eukaryotic cell nucleus, and prior to 1952 it was unknown which molecule type was responsible for passing genetic information between generations. Hershey and Chase designed an experiment to test whether proteins or DNA is the hereditary material.

Outline the procedure of the Hershey and Chase experiment.
AHL A1.2.14— Evidence from the Hershey–Chase experiment for DNA as the genetic material.
Hershey and Chase (H&C) performed their experiments on viruses that infect bacteria called bacteriophages. They incorporated radioactive isotopes of phosphorus and sulfur into phages. DNA contains phosphorus, but not sulfur, whereas protein contains sulfur, but not phosphorus. Therefore, when H &C marked phages with radioactive isotopes of those elements, they placed separate, distinguishable tags on the protein and DNA parts of the phages.
They allowed the phages to replicate by infecting bacteria. By tracking the location of the radioactive tags, H&C showed that phages only injected their DNA into host bacteria, and that the DNA served as the replicating genetic element of phages. The phages did not inject their protein coats into the bacteria; the protein coats remained outside the bacteria adhered to the bacterial membranes.
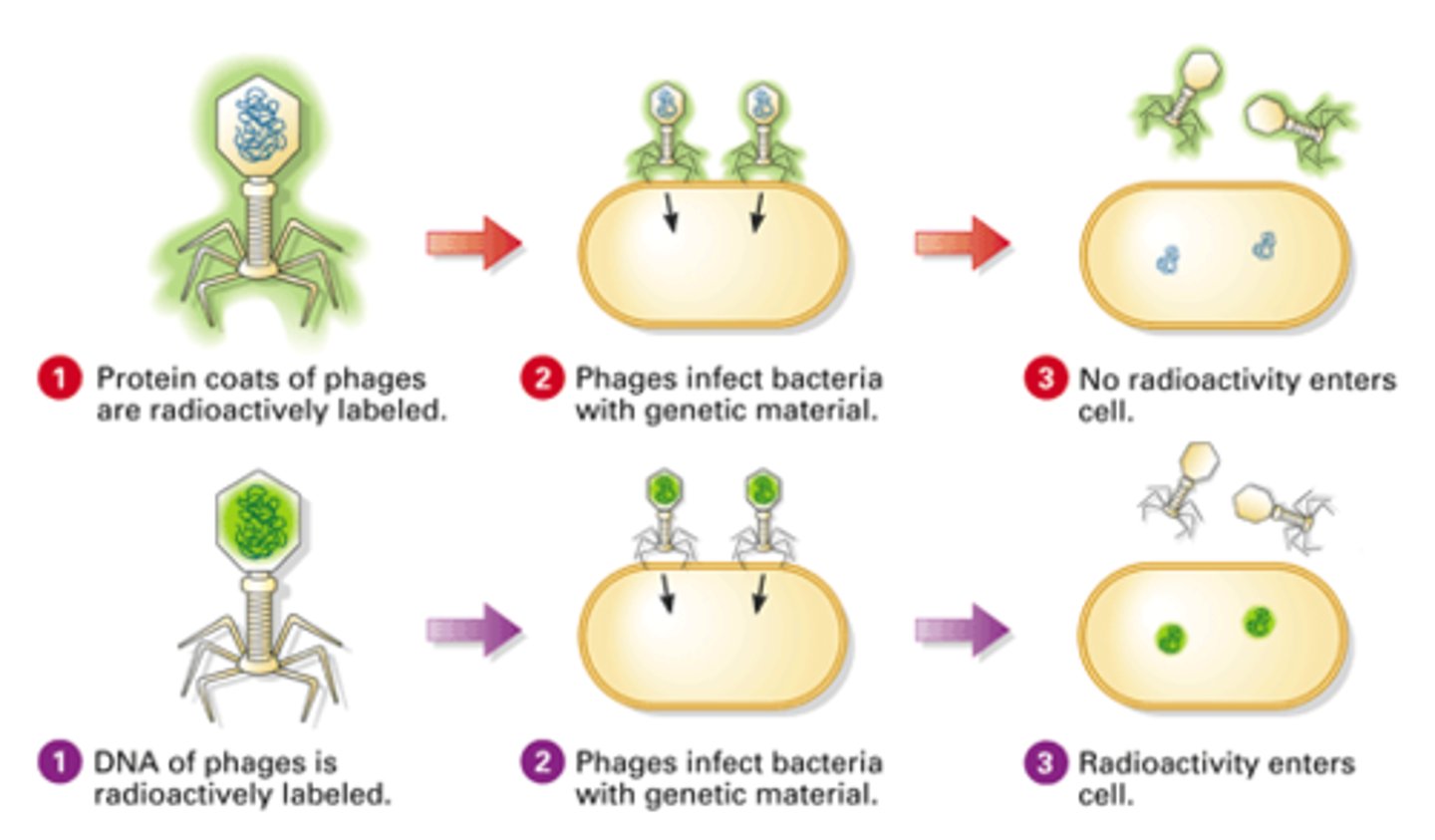
Explain how the results of the Hershey and Chase experiment supported the notion of nucleic acids as the genetic material.
AHL A1.2.14— Evidence from the Hershey–Chase experiment for DNA as the genetic material.
The molecule of heredity must be pasted from generation to generation. When Hershey and Chase demonstrated that radioactively tagged DNA is present across generations of bacteriophages and that radioactively tagged protein is not, they demonstrated that nucleic acids must be the genetic material.
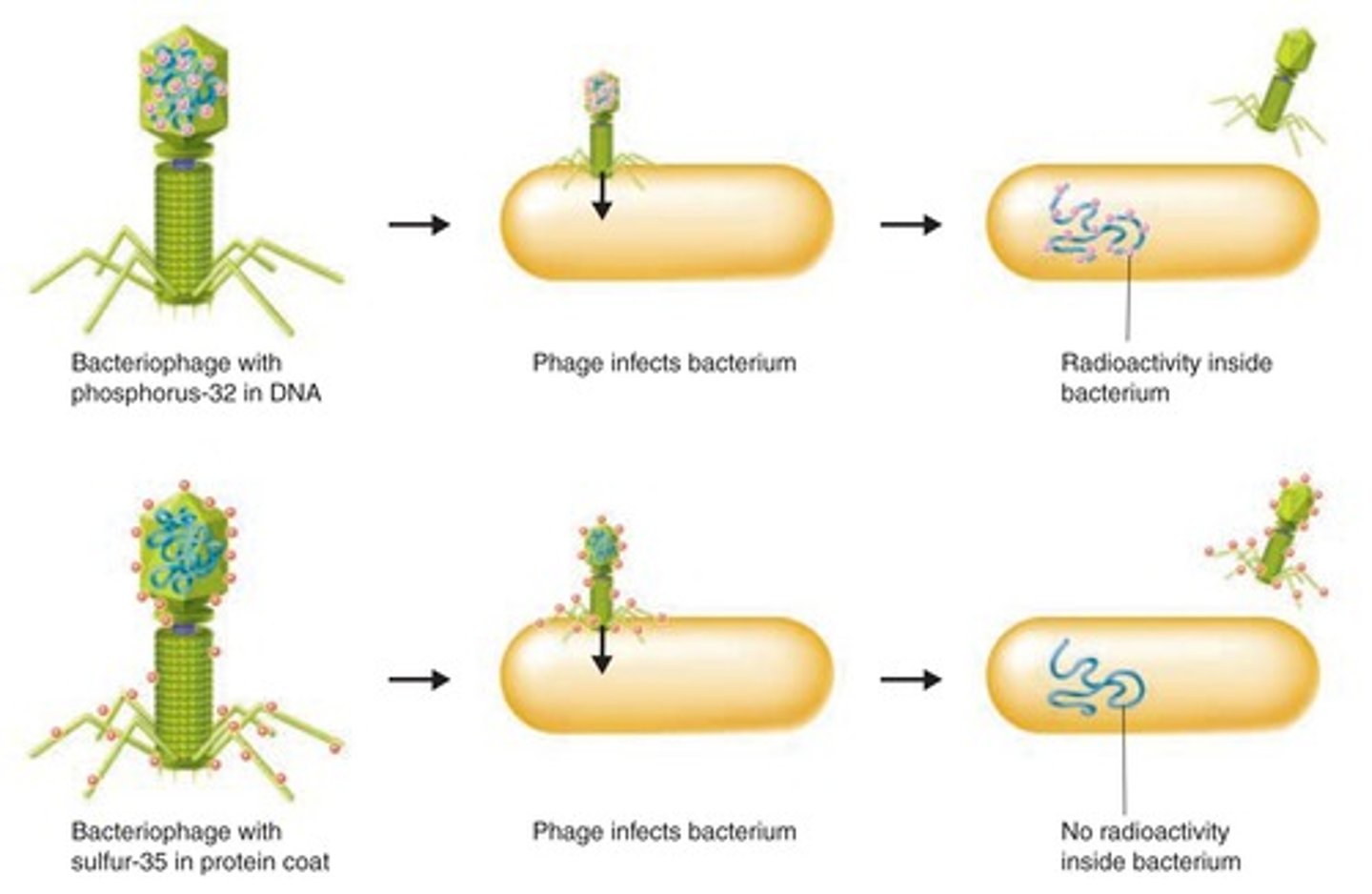
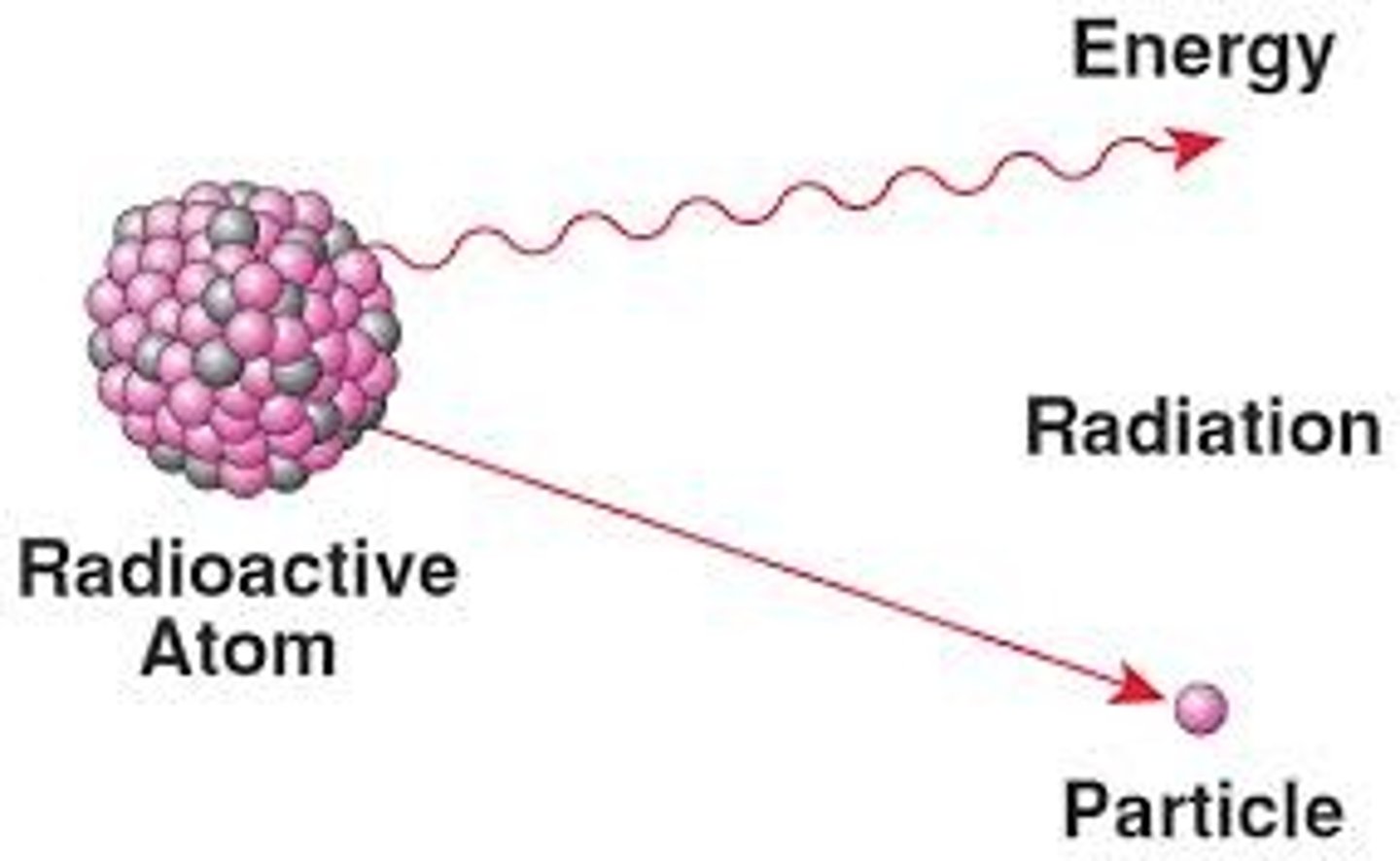
Outline the use of radioisotopes as research tools.
AHL A1.2.14— Evidence from the Hershey–Chase experiment for DNA as the genetic material.
Hershey and Chase relied on radioactive isotopes of phosphorus and sulfur in their experiment to determine that DNA is the genetic material. Although they had been used on a small scale prior, after World War II the U.S. Atomic Energy Commission began mass-producing radioisotopes, sending shipments of radioactive materials to scientists.
Because they give off energy as they convert to a more stable form, the isotopes allow biologists to track molecules as they move through biological systems.
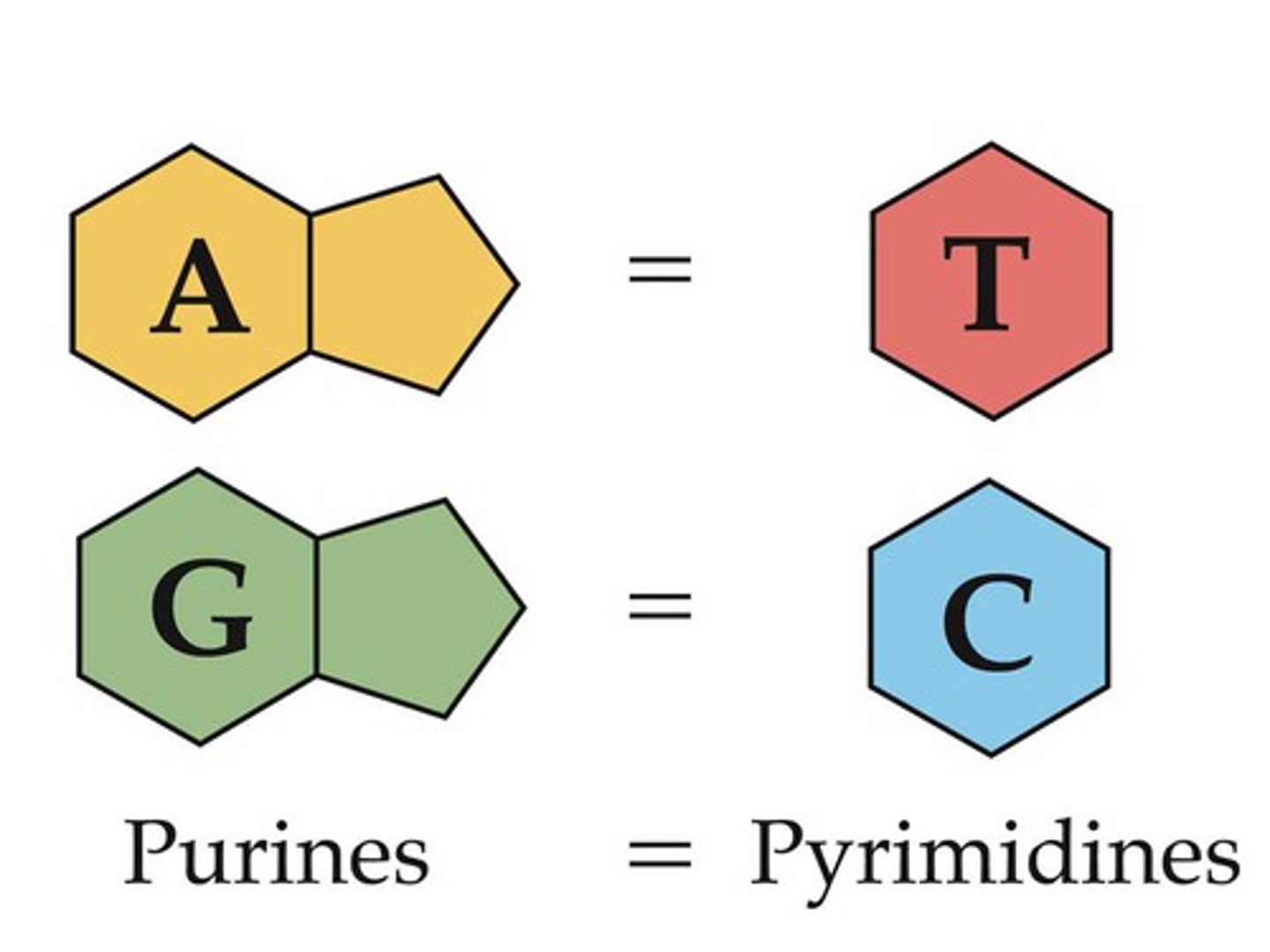
Describe the implications of Chargaff’s data that showed a 1:1 ratio of purine to pyrimidine in a sample of DNA.
AHL A1.2.15— Chargaff’s data on the relative amounts of pyrimidine and purine bases across diverse life forms.
Chargaff's research revealed the percentage of each base (A, T, C, G) found in different species DNA. He determined that there are equal numbers of A and T bases and G and C bases in a DNA sample, resulting in a 1:1 ratio of purines to pyrimidines in DNA.
Chargaff did not use inductive reasoning to then infer the pattern that A binds to T and C binds to G. The complementary base pairing rule was inferred by Watson and Crick.
Explain the role of falsifiability in determining the structure and function of DNA.
AHL A1.2.15— Chargaff’s data on the relative amounts of pyrimidine and purine bases across diverse life forms.
To falsify means that a statement, theory or hypothesis is proven to be wrong.
Because he found that the amount of one base wasn't equal to the amount of the three other bases , Chargaff's data falsified the earlier hypothesis that DNA had a tetranucleotide structure. In this proposed model of DNA, the four bases occur in equal amounts in four strands arranged with the bases facing outward.
How do carbon compounds aid the evolution of cells:
Carbon compounds to form biological molecules
How do monomers aid the evolution of cells:
Molecules capable of self-assembly to form polymers from monomers
Replication aiding evolution of cells:
The ability to replicate to form more cells
How does catalyst of chemical reactions help the evolution of cells?
Catalysis of chemical reactions to speed them up, control them and allow the formation of simple organic molecules from inorganic ones.
Why is inheritance required for the evolution of cells?
Molecules with the ability to self-replicate to allow for inheritance and the persistence of successful variants
How does the evolution of cells require compartmentalisation?
Compartmentalisation from the outside environment to keep useful molecules in proximity and allow a unique internal chemistry.
What structure allows compartmentalisation?
Plasma membrane
What are two functions that ribosomes are capable of?
Able to self-replicate and catalyse reactions.
How do RNA self-replicate?
Many viruses contain RNA which is replicated using protein based enzymes. RNA molecules that can synthesise others have also been artificially produced.
How does RNA catalyse reactions?
RNA molecules called ribozymes found in ribosomes catalyse the formation of peptide bonds between amino acids during protein synthesis
What is spontaneous generation?
The idea that complex life could arise immediately and continually from non-living materials.