MODULATION
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
Discuss the presence and need for inhibitory neurons in brain circuits using an example seen in the body
Inhibitory neurons play a crucial role in the modulation of neuronal circuits by preventing excessive excitatory signals
Inhibitory neuron causes hyperpolarisation potential
This can remove the ability of a number of excitatory inputs to allow the neuron to reach threshold and fire an action potential
e.g. muscle spindle reflexes (one antagonist muscle gets inhibited)
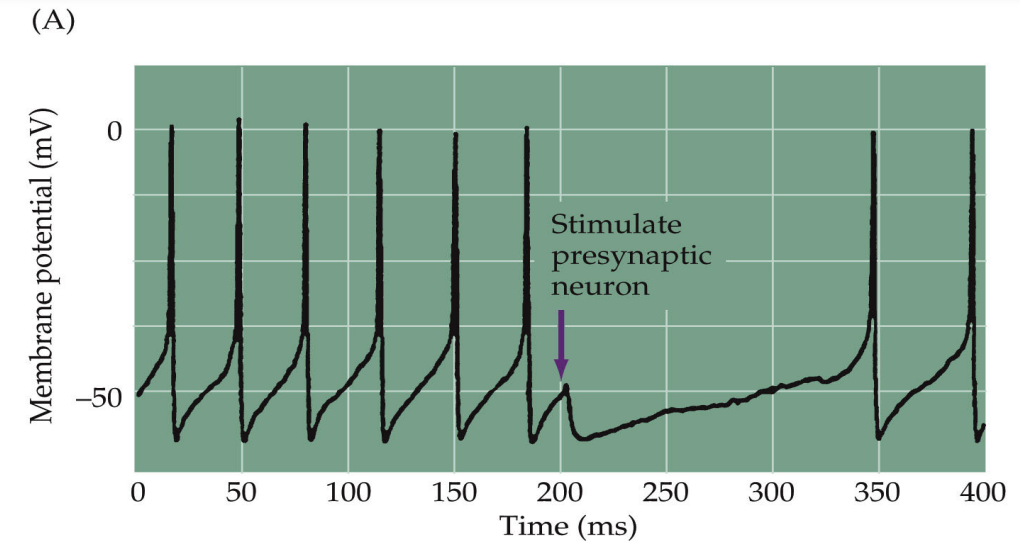
What are the two key fast inhibitory neurotransmitters?
GABA (Gamma-Aminobutyric Acid): The major inhibitory neurotransmitter in the CNS.
Glycine: Another key inhibitory neurotransmitter, particularly in the spinal cord.
Functional Properties: GABA A and Glycine receptors act as chloride channels, influencing neuronal excitability
Explain how GABAergic neuron signalling works (same as glycine neuron signalling)
GABA is concentrated in the VIATT (vesicular amino acid transporter), when there is an influx of calcium due to an action potential, vesicle fuses with the presynaptic membrane and GABA gets released into synaptic cleft
GABA interacts with the GABA receptors (ion channel) on the post synaptic membrane
Explain how GABA gets recycled/removed from the synaptic cleft
GAT (GABA transporter) rapidly removes GABA from the synaptic cleft
Transport either into glial cells to be metabolised or recycled in the presynaptic cell for use as a neurotransmitter
What is the precurser for GABA?
The precursor for GABA is glutamate, the principle fast excitatory neurotransmitter
Most post-synaptic neurons have both glutamate and GABA receptors
Thus, specificity between glutamate and GABA is ESSENTIAL
Glutamic Acid Decarboxylase (GAD): Converts glutamate to GABA using pyridoxal phosphate (also uses a coenzyme called pyridoxal phosphate) in the presynaptic terminal
How does an AMPA receptor differentiate between glutamate and GABA?
Amino acids that form the binding pocket in the receptor are positioned perfectly for the oxygen to interact with glutamate in it's most relaxed form
Even molecules with minor structural differences will not be able to bind and activate the receptor
Explain how GABA and glycine receptors work for inhibition in terms of ion movement.
Through chloride or potassium ions
The equilibrium potential for chloride ions is very close to the resting membrane potential of -65mV, so the electrochemical gradient determines the direction of sodium movement
This means chloride ions can effectively stabilize the membrane potential when sodium channels are open (movement of chloride ions into the cell), preventing excessive depolarization and maintaining neuronal inhibition.
Inhibition can also occur through increased potassium conductance
Is GABA always inhibitory?
No, GABA is not always inhibitory
During the maturation of the nervous system, GABA changes from excitatory to inhibitory
Now the high intracellular cellular concentration of chloride ions means the equilibrium potential of the chloride will be higher (less negative) than the resting membrane potential, meaning that if you open the chloride channel, chloride will leave the neurone, and the neuron will depolarise, meaning the chloride becomes excitatory.
What modulates resting membrane potential in neurons?
Synaptic inputs modulate resting membrane potential to take it closer to threshold through depolarization/excitation or further away through hyperpolarization/inhibition. This leads to changes in graded membrane potential.
What are the three classes of neurotransmitter?
Small-Molecule Neurotransmitters
Examples
Purines: e.g. ATP, Amino Acids: Glutamate, GABA, Glycine.
Biogenic Amine Neurotransmitters
Examples
Biogenic Amines: Catecholamines (Dopamine, Norepinephrine, Epinephrine), Indoleamine (Serotonin), Histamine.
Peptide Neurotransmitters
Serve specific functions in neurotransmission, work via metabotropic receptors
What types of synaptic inputs are there that can modulate membrane potential?
Synaptic inputs can be mediated by chemical or electrical connections
Chemical synapse
Majority of synapses
Release a chemical neurotransmitter (from vesicles into synaptic space) in response to an action potential
Electrical synapse
Gap junctions enable direct ionic passage between neurons, altering postsynaptic potentials.
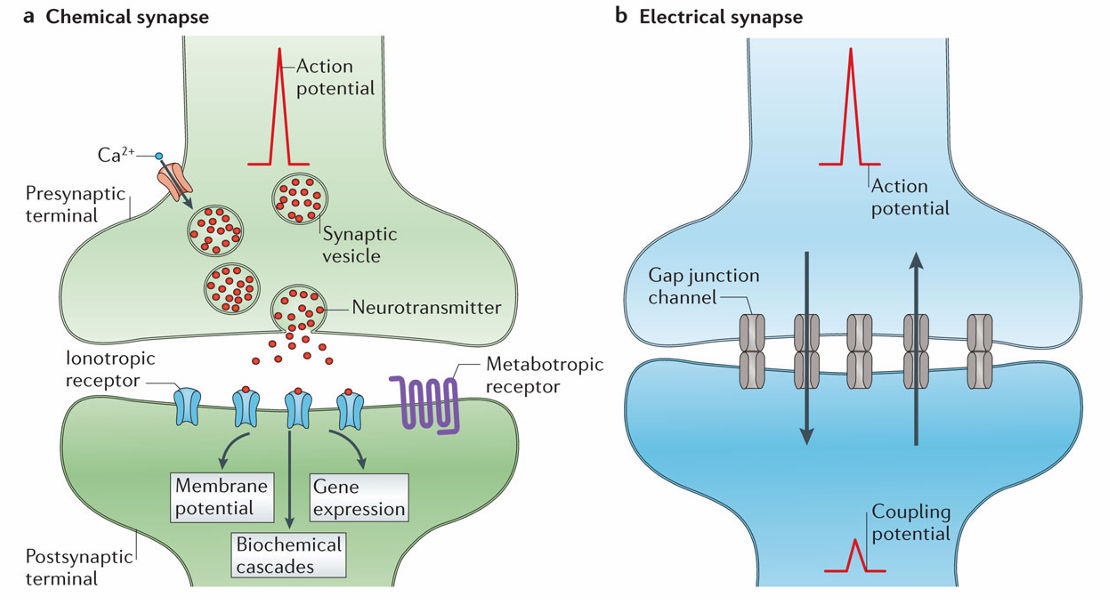
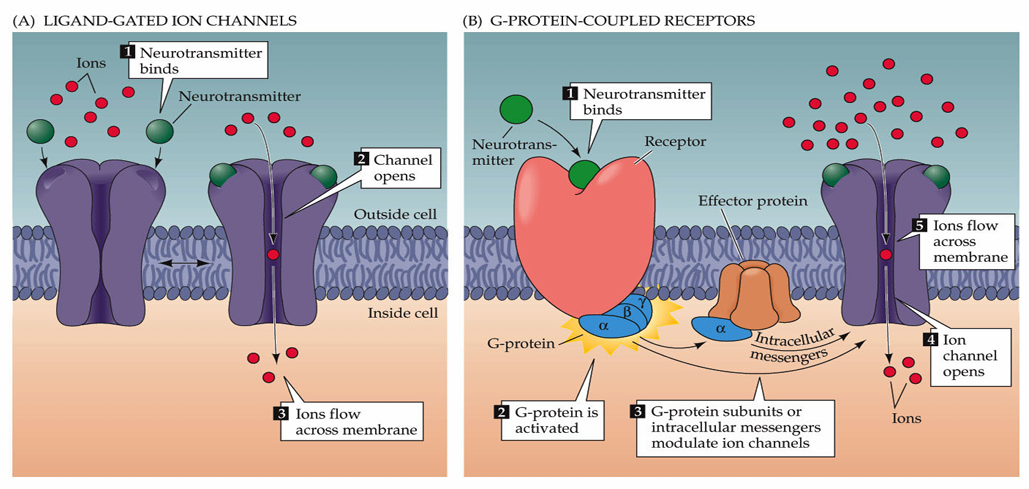
Identify and describe the two types of neurotransmitter receptors
Ligand-Gated Ion Channels (Faster, Ionotropic Receptors)
Neurotransmitter binding opens ion channels, allowing ion movement across the membrane.
The channel will have selectivity but the direction of flow of ions will depend on the electrochemical gradient
These are allow neurotransmitters to be fast as they are almost like an on/off switch
G-Protein-Coupled Receptors (Slower, Metabotropic Receptors)
Neurotransmitter binding to the receptor activates G-proteins, modulating ion channels
Most neurotransmitters work via both ionotropic and metabotropic receptors but metabotropic receptors are slower and allow for modulation
What is the principal fast excitatory neurotransmitter within the mammalian central nervous system?
Glutamate
What are the three principal glutamate ionotropic receptors?
AMPA, NMDA & Kainate each having different pharmacology and actions
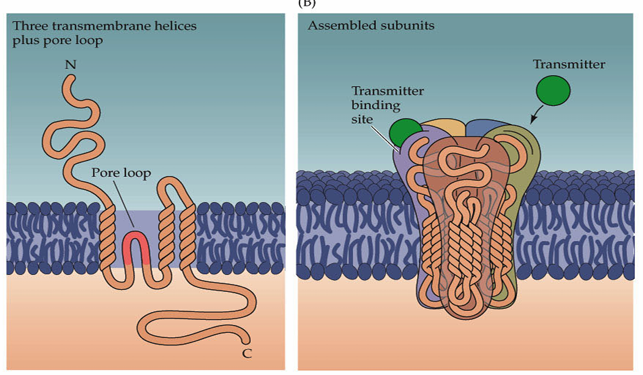
What is the basic structure of glutamate ion channels?
The channels are made up of subunits which have three transmembrane-spanning domains and a re-entrant loop. Most models predict 4 subunits making up an ion channel. There is also an extracellular amino terminus and an intracellular carboxy terminus.
Different receptors are made up of differing combinations of subunits, allowing them to have different characteristics
How does the AMPA glutamate receptor work?
Metabotropic receptor - the ion channel opens when the glutamate neurotransmitter binds to the receptor, allowing ions to flow through
This channel is a non selective cation channel, meaning it allows equal flow of sodium and potassium
Sodium will flow into the cell predominantly, because there is a high electrical and chemical gradient for sodium (interior is negatively charged)
This influx of sodium leads to the depolarization of the interior of the cell (less negative)
As a response the excitatory post synaptic current increases
How does the NMDA glutamate receptor work?
Glutamate neurotransmitter binding to the NMDA receptor opens a channel that transmits Ca2+, Na+ and K+
Has a Mg2+ ion that blocks the ion channel so nothing can pass through
Depolarization of the neuron, leads to repulsion of the Mg2+ ion away from the site, so the ion channel can open and ions can flow through
Thus, a NMDA glutamate receptor needs both ligand binding and a change in voltage to allow the flow of ions through the channel
A crucial difference is that the NMDA receptor leads to the increase of intracellular calcium which is critical for many processes
Explain the process of coupled AMPA and NMDA glutamate receptors.
AMPA receptor activation can happen alone without NMDA
NMDA receptor activation requires activation of AMPA first
Glutamate binds to the AMPA receptor, raising the excitatory post synaptic current and depolarisation of the interior of the cell (rapid)
This enables the glutamate binding to the NMDA receptor to open the channel, as well as the change in voltage allowing the Mg2+ to move out of the way (more prolonged), allowing the ion channel to open
How is glutamate neurotransmitter produced in presynaptic terminals?
Glutamine converted to glutamate via glutaminase in presynaptic terminals.
It is then packaged in synaptic vesicles by VGLUT (vesicular glutamate transporter) followed by release at synapses, through fusion of the vesicles with the membrane.
How can glutamatergic neurons maintain high firing rates and fidelity and explain how these processes occur?
Rapidly removal of glutamate from the synaptic cleft, enables high fidelity of the signalling
Glutamate rapidly removed from synaptic cleft by transporters (EATT, excitatory amino acid transporters
The predominant form of EATT is present in the cell membrane of the glial cells, particularly astrocytes
Glutamate can be removed, recycled, repackaged and reused
EATT, excitatory amino acid transporters take the glutamate in to the intracellular space of the astrocyte, where it is inactivated by glutamine synthetase and is recycled to glutamine, which is inactive so can be released into the extracellular space and transported by EATT back to the presynaptic space
Once in the presynaptic terminal, glutamine can then be converted to glutamate by glutaminase, so it can be repackaged and used for another synapse
What fast ionotropic neurotransmitter is used for communication between motor neurons and muscles and what is it’s ionotropic receptor called?
Acetylcholine which uses nicotinic receptors
What is the structure of nicotinic receptors?
They are non-selective cation channels constructed from pentamers of four transmembrane domain subunits.
What is the slow metabotropic receptor used for the neurotransmitter acetylcholine?
Muscarinic receptor (G-coupled protein receptor)
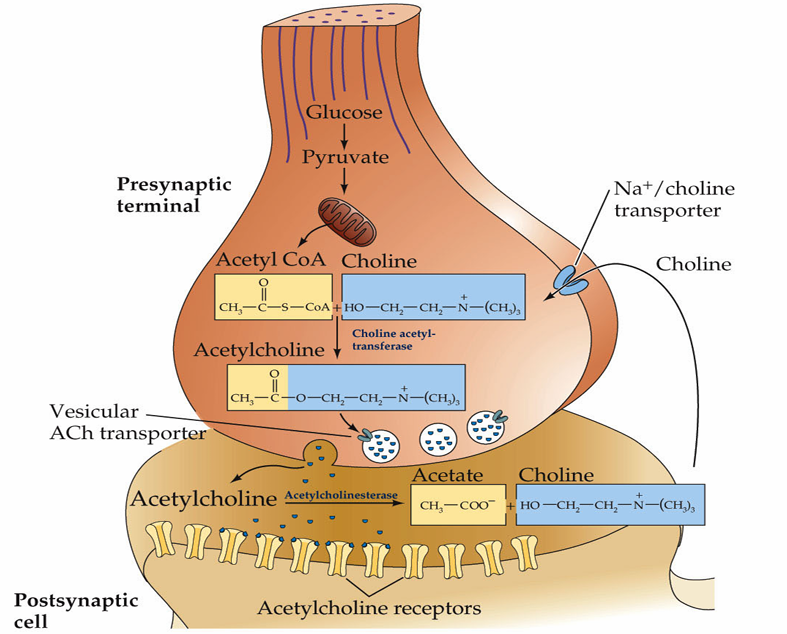
How is acetylcholine cleared from the synaptic cleft?
Degradation. Acetylcholine is rapidly metabolized in the synapse by the enzyme, acetylcholinesterase
In contrast to glutamate, acetylcholine is not rapidly removed from the synaptic cleft by transporters, it is instead broken down by acetylcholinesterase which reduces it to acetate and choline
From here the acetate is broken down and the choline is recycled via the Na+/Choline transporter that facilitates its reuptake into the presynaptic neuron.
How do electrical synapses work?
Electrical synapses enable ionic currents to pass directly between neurons, influencing membrane potential through gap junctions.
For electrical synapses, there is a physical connection between the intracellular sides of the pre and post synaptic elements, this is via gap junctions called connexins
They can also be modulated to be opened or closed, just because they are open doesn’t mean they always allow for the flow of ions
What is the direction of ion movement in gap junctions?
Ion movement may be bi-directional in gap junctions
An input into one cell, leads to a change in the membrane potential of that cell, thus ions move following the electrochemical gradient into the other cell
These gap junctions can also be used for the movement of other small molecules such as ATP or calcium
Many ionotropic receptor ion channels are not selective for a particular ion and do not determine the direction of ion movement - what does?
The effect of opening the channel is dependent on:
membrane potential
ion concentrations
What are the molecular mechanisms underlying the resting membrane potential?
The resting membrane potential (RMP) is typically around -65 mV, generated through a combination of
passive leak channels for K+ and Na+ ions allows cells to flow along concentration gradients
Na+/K+ pump (which actively transports Na+ out of the cell and K+ into the cell against concentration gradients)
The separation of charges across the neuronal membrane creates a state of potential difference crucial for action potentials.
How do calcium transients allow for action potential measuring?
Calcium transients are short-term increases in intracellular calcium ion concentrations that occur in response to various cellular stimuil:
When an action potential occurs, voltage-gated calcium channels open, calcium ions (Ca2+) to enter the cell.
This influx of calcium can act as a surrogate signal for action potentials, indicating that an action potential has occurred even if the exact action potential is not directly measured.
What is the difference between resting, graded and action potential?
Resting Potential
The membrane potential when a neuron is at rest. Typically around -65mV.
Graded Potential
Changes in membrane potential based on the stimulus received by the neuron.
Action Potential (AP)
An event that may lead to synaptic activity on the subsequent neuron, requiring specific conditions to occur.
What influences the movement of ions across the cell membrane?
Ion movement is influenced by concentration gradients and difference in electrical potential across the membrane.
Concentration gradients:
Movement of ions from regions of high concentration to low concentration
Electrical forces
Opposite charges attract, while like charges repel, leading to ion movement towards electrodes based on electrical potential. Depends on electrical potential and electric conductance.
What is the rough distribution of sodium, potassium and chloride ions across the membrane?
Potassium is more concentrated on the inside of the cell (1:20) so leaks out of the cell
Sodium is more concentrated on the outside of the cell (10:1) so leaks into the cell
Chloride is more concentrated on the outside of the cell (15:1) so leaks into the cell
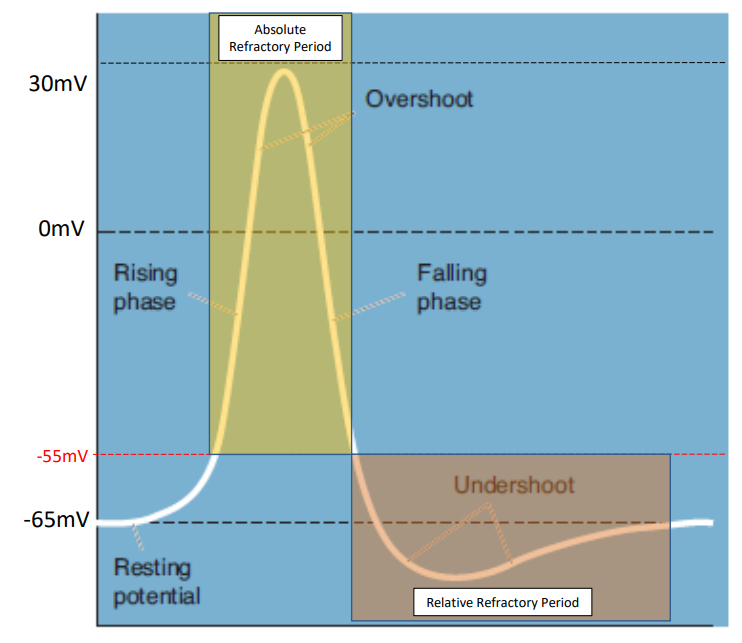
Explain how an action potential occurs
Once membrane potential reaches threshold (~-55mV):
voltage gated sodium channels will open, allowing sodium to flow into the cell causing depolarization (rise in membrane potential). These channels close after 1ms.
During depolarisation, voltage-gated potassium channels open, which leads to repolarization as potassium exits the cell. This repolarises the neuron, bringing it back down to resting membrane potential.
There will be a slight overshoot of voltage which is equilibrated by leak channels. (called the undershoot phase)
What are the absolute and relative refractory periods?
Absolute Refractory Period: No new action potential can be initiated. (occurs above 55mV)
Relative Refractory Period: A stronger than usual stimulus is necessary to initiate an action potential. (occurs in the undershoot phase)
How do action potentials propagate?
Action potentials propagate along the axon due to the high density of voltage-gated channels in the axon and at nodes of Ranvier.
As the action potential travels across the axon there is depolarisation of segments of the cell that creates a flow of charge changes along the axon
The flow of sodium into the cell from one volage gated sodium channel creates a change in membrane potential enough so that the adjacent one can open and so on
The action potential can't go backwards because of the efflux of potassium out of the neuron and the inactivation of previously opened sodium channels.
What is saltatory conduction?
Rapid conduction in myelinated axons, where action potentials jump from node to node
Where in an axon is an action potential generated?
Action potentials are generated at the axon initial segment (AIS), heavily populated with voltage-gated channels.
The AIS is just below the soma and the hillock
What key proteins are involved in action potential generation which have high expression in the AIS?
beta4-Spectrin and Ankyrin-G, which facilitate the clustering of channels at the AIS and nodes, critical for effective action potential transmission and generation
What areas of the nodes of ranvier have expression of what channels?
The nodal section has a high expression of voltage gated sodium channels
The juxtaparanodal and paranodal regions have a high expression of voltage gated potassium channels
How do different neurons have variations in the way they generate action potentials?
Different neurons in the brain have different expressions of different isoforms of voltage gated sodium and potassium channels
What is the purpose of metabotropic receptor pathways and describe briefly how these work?
G-coupled and metabotropic pathways are very important in the modulation of neuronal activity
Signal binds to the receptor
Receptor activated
Change in function occurs
What are the two main classes of G-protein coupled receptors?
Heterotrimeric
Chemical signalling molecule binds to the receptor, interacting with the heterotrimeric G-protein with 3 subunits that in response to the conformational changes of ligand binding, dissociates and alters the function of the effector protein and different intracellular signalling pathways
Monomeric
are able to interact with GTPases and can affect neuronal function
How do G-Protein Coupled Receptors work?
Sitting in the membrane are alpha, beta and gamma subunits, lipid modified so they can associate with the membrane
In the resting state (inactive) GDP is bound
When the agonist binds to the receptor, it stabilises the receptor in a conformation that binds the alpha subunit
The alpha subunit then changes its conformation and the GDP is removed and guanine triphosphate (GTP) associates, disassociating the alpha from the beta-gamma subunit
The alpha and the beta-gamma can then interact with effector proteins that generate a response such as intracellular signalling molecules such as cAMP, change conductance of calcium or potassium channels which affect neuronal function
Dephosphorylation of the GTP also occurs so the alpha and beta subunits can recombine and be ready to be activated again
Explain the main benefit of GPCRs?
Massive amplification
Low concentration of ligand and receptor can have large effects
A receptor interacts with a single ligand, but can interact with multiple G-proteins, which each interact with an effector protein (e.g. Adenylyl cyclase) which can then have an enzymatic effect to produce MULTIPLE intracellular signalling molecules (cyclic AMP), which can interact with other molecules, having a variety of different effects
Whare are the 3 Main G-Protein Types?
Galpha/Gaplha s for stimulation (depolarisation)
Galpha i for inhibition (hyperpolarisation)
Galpha q ultimately increasing cell activity
How do the Galpha/Gaplha s receptors work?
Galpha/Gaplha s for stimulation (depolarisation)
Interacts with and stimulates adenylyl cyclase
Increases cyclic AMP
which increases protein kinase A
increasing protein phosphorylation which means the cell activity will increase
How do the Galpha i receptors work?
Galpha i for inhibition (hyperpolarisation)
Interacts with and inhibits adenylyl cyclase
Inhibits cyclic AMP
which inhibits protein kinase A
decreasing protein phosphorylation which means the cell activity will be decreased
How do the Galpha q receptors work?
Galpha q ultimately increasing cell activity
Binds to GTP which leads to an interaction with the effector protein phospholipase C
which catalyses the reduction of phosphoinositol biphosphate, a membrane lipid protein leading to the production of:
inositol trisphosphate (IP3), which binds to intracellular receptors that lead to the release of calcium from the endoplasmic reticulum
diacylglycerol (DAG) which can interact with protein kinase C another effector which when combined with calcium can produce more effects
This increases the protein phosphorylation and activates the calcium-binding proteins
How do intracellular signalling molecules directly interact with ion channels to alter neuronal function?
Neurotransmitter receptors: neurotransmitter such as glutamate binds to the receptor enabling ions to flow through
Calcium ion activated potassium channel: uses calcium as a signalling molecule to change the conductance of potassium
Cyclic nucleotide gated channel: Cyclic AMP can also interact with an ion channel leading to changes in conductance of select ions such as sodium and potassium
What are the 8 neurotransmitter receptor classes that interact with metabotropic receptors?
Glutamate
GABA B
Dopamine
Adrenaline and Noradrenaline
Histamine
Serotonin
Purines
Muscarinic
What is the post-synaptic effect of metabotropic receptors?
Post synaptic metabotropic receptors act via trimeric G proteins which activates a pathway to alter ion channels and ultimately membrane potential
What changes other than to membrane potential can receptors have?
Metabotropic and ionotropic receptors can cause changes in intracellular calcium and other messengers that don't directly and immediately impact membrane potential, but make changes that alter neuronal function via changes in gene expression
That change in gene expression can have a range of vast impacts
How can presynaptic actions modulate the probability of release of neurotransmitters?
Presynaptic facilitation
Presynaptic inhibition
Feedback regulation
How does presynaptic facilitation modulate the probability of release of neurotransmitters?
serotonergic neuron releases serotonin to a serotonin receptor on the presynaptic cell
this shuts down the potassium current, which leads to further depolarisation of the nerve terminal
making it more likely to release neurotransmitter in response to an action potential
How does presynaptic inhibition modulate the probability of release of neurotransmitters?
GABAergic neuron interacts with a GABA B receptor
this opens the potassium channel leading to hyperpolarisation of the terminal
making it less likely to release neurotransmitter
This can also be done with the ionotropic GABA A receptor channel which leads to further hyperpolarisation by a chloride channel
How does feedback regulation modulate the probability of release of neurotransmitters?
neuron releases noradrenaline into the post synaptic space
noradrenaline can then also bind to a receptor on the presynaptic neuron (alpha adrenoceptor) that can in turn alter activity of calcium channels
Decreasing the entry of calcium will decrease the probability of release, thus inhibiting the presynaptic neuron, modulating itself
What monoamines make up the catecholamines of the aminergic systems?
dopamine, noradrenaline and adrenaline, which each have multiple monotropic receptor subtypes
How are the aminergic catecholammines synthesised?
These neurotransmitters are produced from tyrosine via an enzymatic cascade
dihydroxy phenylalanine is produced from tyrosine via tyrosine hydroxylase
Dopamine is produced by DOPA decarboxylase
Noradrenaline is produced by dopamine-beta-hydroxylase
adrenaline is produced by phenylethanolamine N-methyltransferase.
How does the production of the catecholamines occur in the presynaptic neuron?
An action potential leads to the influx of calcium into the neuron which stimulates the production of monoamines
Calcium works on protein kinase which phosphorylates tyrosine, produces more dopamine to be packaged in the vesicles to be released into depolarisation of the presynaptic terminal
For the other monoamines, dopamine beta hydroxylase and PNMT are present within the vesicles where they convert the dopamine into noradrenaline or adrenaline
What is the transporter that puts the catecholamines into the vesicles called?
Vesicular monoamine transporter (VMAT)
How are these catecholamines cleared and recycled from the synaptic cleft?
Once released into the synaptic cleft, the amines are either taken back into astrocytes or the presynaptic terminal where they are broken down by either monoamine oxidase (MAO) or catechol-O-methyltransferase (COMT)
The uptake is assisted by a dopamine transporter (DAT which is a sodium dependent co-transporter) or the noradrenaline co-transporter which is more specific and can also take dopamine
What is the structure of GCPRs?
They all have 7 transmembrane spanning domains and are all associated with G proteins