AP Biology Unit 1 Chemistry of Life Daily Videos
1/82
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
83 Terms
The subcomponents of biological molecules determine what.
The subcomponents and the structure of biological molecules determine the properties of that molecule.
What subcomponents are water molecules composed of
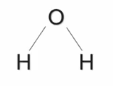
Water is composed of 2 main elements, oxygen and hydrogen, in a 1:2 ratio respectively (H2O).
And in these water molecules bond with covalent bonds, however became oxygen is more electronegative compared to hydrogen, it results in an unequal sharing of electrons between oxygen and hydrogen.
This covalent bonding can result in polarity when there are differences in atomic electronegativity, causing the hydrogen to have a partial positive and the oxygen to have a partial negative charges.
Covalent bond
Is a type of chemical bond where atoms share elections to achieve a more stable electron configuration, instead of one atom transferring electrons to another, as in an ionic bond.
Because oxygen is more electronegative compared to hydrogen, it results in the formation of polarity due to the unequal sharing of electrons between oxygen and hydrogen.
Polarity
The uneven distribution of electrons within a molecule, creating partial positive and negative ends, like a tiny magnet.
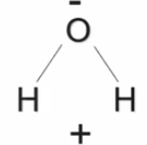
Hydrogen bond
A ___________ ____ is a weak bond interaction between the negative and positive regions of two separate molecules.
Water can form _________ ____ with other water molecules or with the same charged molecules.
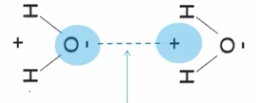
Cohesion
When two of the Same molecules form hydrogen bonds with each other this is called ________.
Adhesion
Is when two different molecules from hydrogen bonds with each other this is called ________.
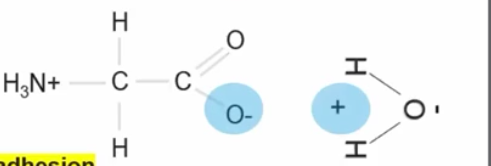
Surface Tension
Is the property of a liquid’s surface that allows it to resist external forces due to cohesive forces between its molecules.
Is the result of increased hydrogen bonding forces between water molecules at the surface.
For water, its polarity and subsequent strong hydrogen bonds create high _______ ______, making its surface behave like a “skin” that allows small, light organisms like water strides to walk on it and helps maintain cell membrane structure.

How is surface tension important to living things
Surface tensions is important for living things because many aquatic plants rest on the surface of water, allowing them to have more access to sunlight for processes like photosynthesis.
Solvency
is the substance, usually a liquid, that dissolved another substance (the solute) to form a solution.
Water is the most important example of a solvent. Because of its polar nature, water is often called the “universal _______,” and its ability to dissolve many different substances Is critical for life.
Water is able to act as a ________ because when substances like NaCL is placed in water, the partially positive hydrogen atoms are attracted to the negative chlorine ions, and the partially negative oxygen atom is attracted to the positive sodium ions.
This attraction pulses the ions apart and surrounds them with a layer of water molecules called a hydration shell, effectively dissolving the salt.
So water’s adhesive property gives water a high ________ ability in its liquid state.
How is water solvency properties essential for life.
Because organism gain key nutrients from their environment, considering that living things are made up of 70% water, dissolved materials in the water allow for easy access by cells.
Water’s unique hydrogen bonding in its different states of matter.
Water’s cohesive property allows for unique hydrogen bond interactions to occur when water is in a solid state, making ice less dense than liquid water.
Water’s cohesive property forces water molecules into a unique, open, crystalline lattice structure when freezing. In this solid state , molecules are held farther apart then they are in the more mobile liquid form. Causing these bonds to be stable, apart from liquid water when hydrogen bonds constantly break and re-form.
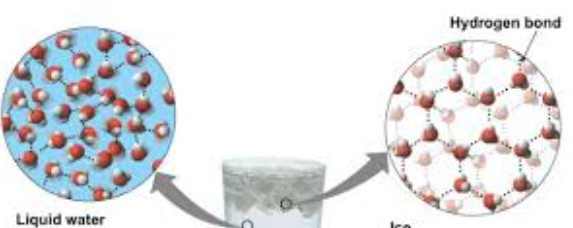
Why is water’s ability to have unique cohesive forces in a liquid verses a solid state of matter important for living systems.
Water’s ability to have unique cohesive forces in a liquid verses a solid states of matter is important for living system because aquatic organisms are still able to live in freezing climates, because the water freeze on the surface, leaving liquid water underneath for those organisms.
Water’s high heat capacity
Is the amount of heat energy a substance must absorb or lost to change its temperatures by 1 degree Celsius.
Water’s cohesive property allows it to above a lot of thermal energy before changing chemical states, resisting sudden changes in temperatures because more energy needs to be directed in order to break the hydrogen bonds.
This is essential to living things because aquatic organisms that rely on their environment to regulate their temperature will dependent on this property of water so they will maintain a stable body temperature regulation.
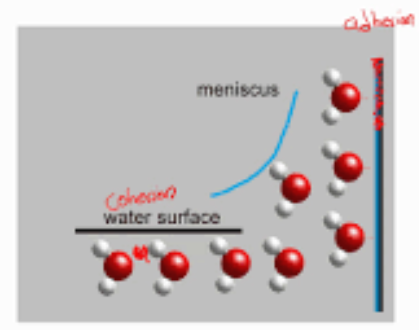
Capillary action
Is the upward movement of liquid withing narrow spaces (like a plant xylem) that defies gravity, driven by the combine forces of adhesion (water sticking to the container walls) and cohesion (liquid molecules sticking to each other).
This is important to
Why do Living systems require a constant input of energy.
Because the law of conservation of energy states that energy cannot be created or destroyed only transformed, living systems follow the laws of energy.
So living systems need a constant input of energy to grow, reproduce, and maintain organization as they can not create their own energy so they rely on their environment to consistently supply them with energy withing the continuous movement of matter in Earth’s biogeochemical systems.
Why do living system require an exchange of matter
Living systems require an exchange of matter because atoms and molecules from the environment are necessary to build new molecules.
As carbon is used to build biological molecules such as carbohydrates, proteins, nucleic acids, and lipids.
Or nitrogen is used to build proteins and nucleic acids.
And phosphorus is used to build nucleic acids and certain lipids (are know as phospholipids, and they are characterized by having a glycerol backbone—is a sugar alcohol that serves as a building block for lipids, while its chemical structure has characteristics of a carbohydrate—, two fatty acids, and a phosphate group that gives them a hydrophilic head)

Carbon being used to build macromolecules
Carbon is used to build macromolecule because its unique atomic structure allows it to form up to form stable covalent bonds with other atoms, including itself. This versatility lets carbon atoms create a stable, complex, and diverse “backbone” of chains, rings, and branched structures that form the basis of essential like molecules like proteins, carbohydrates, nucleic acids, and lipids.
Characteristics of Carbon
Carbon can bond to other carbon atoms creating carbon skeletons to which other atoms attach.
Carbon skeletons allow for the creation of very large and complex molecules.
Carbon containing molecules can be used to store energy, and carbon containing molecules can be used to form basic cell structures like membranes
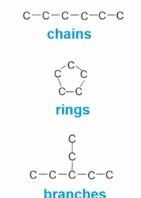
The different carbon backbone structures.
Carbons can form chains, rings, and branches which are some ways that similar macromolecules can differentiate in their structure and therefore their function.
The important properties of Monomers
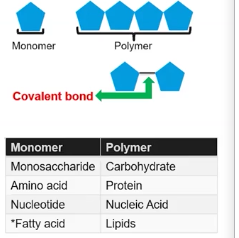
Some important characteristics of monomers is they are chemical subunits used to create polymers (is a macromolecules made of many monomers).
A covalent bond is formed between two interacting monomers, and monomers have specific chemical properties that allow them to interact with one another.
Moreover polymers are specific to the genera of monomers they consist of as they derive their proteins from the monomers they are made up of.
Some examples would be a Monosaccharides monomer would make up a carbohydrate polymer, and amino acid monomer would make up Protein polymers, and a Nucleotide monomer would make up Nucleic Acid polymer, and a fatty acid monomer would make up a lipids polymer. However member lipids don't have a specific monomers.
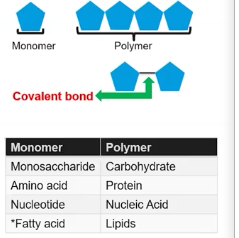
Monomer
It is a small molecule that serves as a “building block” that can chemically bond with other identical or similar small molecules to form a much large molecule called a polymer.
Some examples of monomer would be a Monosaccharides that would form carbohydrate polymers, a nucleotide that would form a nucleic acid polymer, or a amino acid that would form a protein polymer, however lipids don't have a specific monomer.
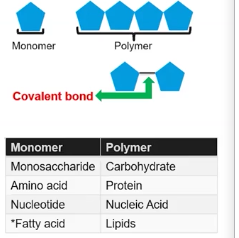
Polymer
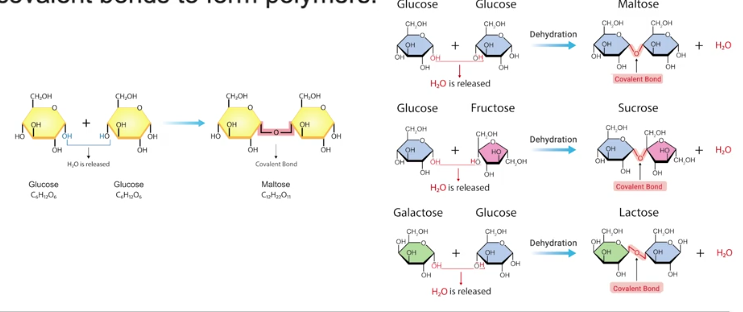
It is a large, complex molecules made up of many smaller, repeating subunits called monomers that are linked together, often through dehydration synthesis reactions.
Dehydration Synthesis reactions
This is a reaction that are used to create marcmolecules and this is when the subcomponents of a water molecule (H and OH) are removed from interacting monomers and a covalent bonds forms between them.
The H and OH join together to form a molecule of water, water is a byproduct of this reaction.
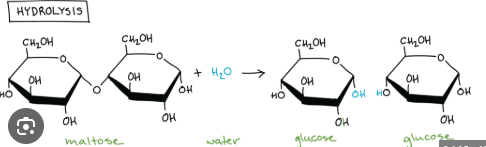
Hydrolysis reaction
This is a reaction in where polymers are hydrolyzed (broken down) into monomers during a hydrolysis reaction.
Covalent bonds between the monomers are cleaved during a _____ ______ as a water molecules is hydrolyzed into subcomponets (H and OH) and each subcomponent is added to a different monomer.
It is important to note that breaking any chemical bond, including the one in a polymer, requires energy. This energy know as activation energy is needed to initiate the reaction. During the reaction, a water molecule is split into a hydrogen ion (H+) and a hydroxly group (OH-). These fragments are added to the large molecule, breaking a covalent bond and separating it into smaller units (monomers)
The H+ and -OH fragment from the water molecule form new, more stable bonds with the monomers. The energy released by the formation of these more stable bonds is greater than the initial energy required to break the polymer bond/
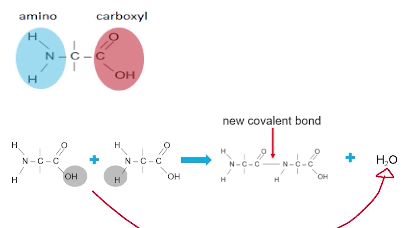
Example of dehydration synthesis creating proteins.
A Protein monomers are called amino acids. And each amino acid has an amino group (NH2) terminus and a carboxyl group (COOH) terminus.
A hydroxide (OH) is lost from the carboxyl group of one amino acid and a hydrogen atom is lost from the amino group of another amino acid.
A covalent bond will form between the monomers in the location where the hydroxide and hydrogen atom were removed
The hydroxide (OH) and hydrogen atom (H) will join forming a water molecule (H2O).
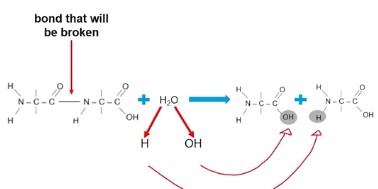
Example of hydrolysis reaction causing amino acids to form from proteins.
Covalent bonds between amino acids can be cleaved, an water molecule is hydrolyzed and each subcomponent of water (H and OH) will be bonded to different amino acids.
The result is separate amino acid monomers.
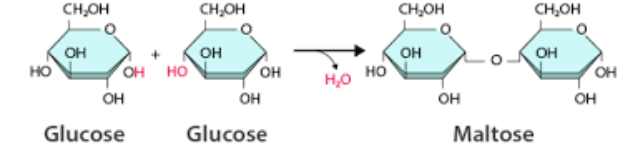
Directionality of the subcomponents in Carbohydrate influence the structure and function of the carbs.
Carbohydrates comprise of liner chains of sugar monomers (monosaccharids/ simple sugars) connected by covalent bonds, and small direction changes in the components of a molecule can results in function differences.
For the most part the change in different direction in the OH group in a simple sugar (monomers) can change the function of the polymers it created.
For example in the monomer glucose (alpha) because the OH group is facing the bottom when they come together to form a polymer it forms starch, which is used for energy storage in plants. (due to causing the different direction of the covalent bond)
However in the monomer glucose (delta) because the OH group is facing the top when they come together to form a polymer it forms cellulose, which is used for structural support for plants. (due to causing the different direction of the covalent bond)
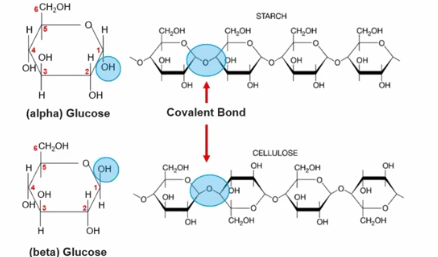
The structer of Carbohydrate polymers which influence its function
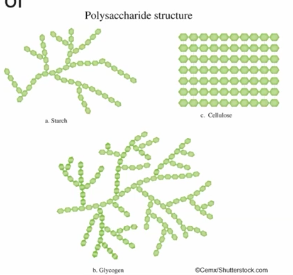
Carbohydrates polymers may be liner or branches and this structure causes the function of the polymer function to be unique despite most of them composing of the same types of elements (C6H12O6 x # of monomers - H2O x # of monomers)
For example starch and glycogen and both assembled from similar forms of glucose, and these glucose monomers are connected by covalent bonds in similar locations and orientations. Which leads to branching structure in both polysaccharides and similar function as starch is a stored form of sugars and energy in plants, whereas, glycogen is a stored form of sugar and energy in animals.
However, cellulose is assembled from a different from of glucose monomer and therefore when those particular subunits are connected, they form long, liner, unbranched chains.And when these linear strands are arranged side by side hydrogen bonding can happen between these strands which will causes the cellulose molecules to cluster together. Therefore the function of cellulose can be providing structure strength in plant cells walls.
Monosaccharides
Is the fundamental, indivisible units of carbohydrates, also called simple sugars, and the serve as essential energy sources end building blocks for more complex sugars like disaccharides and polysaccharides.
And their monomer are connected by covalent bond to form polymers.
Key example include glucose, fructose, and galactose, which are water-solube and often colorless, they are often characterized by their chemical formula (CH2O).
Polysacchardies
Are complex carbohydrates made of many simple sugar units, known as monosaccharides, linked together by glycosidic bonds (covalent bonds).
They sever crucial roles as energy stores and structural compent in living organisms, with common examples including starch (primary energy source for plants) and glycogen (used for short-term energy storage in animals), and cellulose (a major structural components of plant cells walls and the most abundant organic molecule on earth), and chitin (a structural __________ found in the exoskeletons of insects and crustaceans)
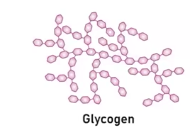
Glycogen
It is a polysacchardies and it main storage form of glucose in animals and it provides quick energy when needed.
It’s main structure is categorized as being highly branched polymer due to its type of glucose monomer, which influenced on the polymer structure and therefore its function.
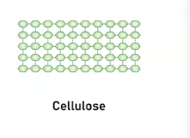
Cellulose
It is a polysaccharides and it is a structural component in plant cells walls, providing rigidity and strength.
It’s main structure is categorized as being a lineal chain due to it type of monsacchardies that influence its polymer structure and therefore its function.
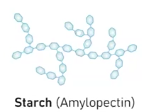
Starch (Amylopectin)
It is a polysaccharide and its a major component starch in plants which has a branched structure for rapid glucose releases.
It’s main structure is categorized as having a branch structure that feature liner segment that twist into double helices, and this is due to the type of monsacchardies that influence its polymer structure and therefore its function.
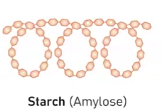
Starch (Amlyose)
It is a polysaccharide and its a major component starch in plants which store energy in long, unbranched chains of glucose.
It's main structure is categorized as having liner chains fold into a double-helix structure, and this is due to the type of monsacchardies that influence its polymer structure and therefore its function.
Lipids
Are typically no polar, hydrophobic molecules whose structure and function are derived from the way their subcomponents are assembled.
And ______ like triglycerides are built by combing glycerol + 3 fatty acids through ester bonds.
Most lipids are nonpolar and hydrophilic because the have long hydrocarbon chains made mostly of carbon and hydrogen, which share electrons evenly and don’t mix well with water as their is no polarity so they can’t for hydrogen bonds with water.
Unlike carbohydrates (which have -1:2:1 ration of C:H:O), ______ have a lot more carbon and hydrogen compared to oxygen. This makes them very energy-rich and non polar (as they are stable since they are not reactive in terms of polarity)
The main functional groups that make up lipids are the Hydrocarbon chain (however not a functional group), that are the no polar backbone, the Carboxyl group ( -COOH), found at the end of fatty acids.
Lipids are mainly used for long-term energy storage (due to the fact it hard to break them up because they don’t have polarity, so that prevent them from breaking up in hydrolysis), making up cell membranes (phosphlipids), and sending signals (steroids, hormones)
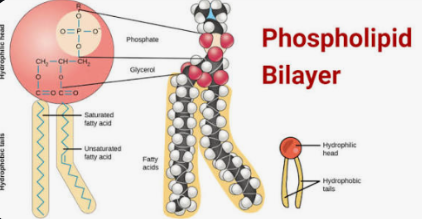
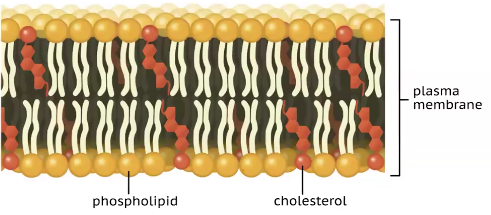
phospholipids
These are lipids that are the expectation to the normal function as they are amphipathic— which means they have a polar, hydrophilic phosphate head, that loves water and a no polar hydrophobic fatty acid tails that avoid water. This dual property makes them unique.
Like other lipids, they have more carbon and hydrogen than oxygen, but the phosphate group adds extra oxygen and makes the head polar.
The main functional groups would be a hydroxyl group (-OH) in where each carbon is bonded to one of these groups in the glycerol, and sometimes in the head of the __________. A phosphate group (-PO4³).
The Carboxl group (-COOH) is found at the end of fatty acids and there is a hydrocarbon chains that are the no polar backbone of the fatty acids.
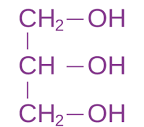
The structure of this is where there is a Glycerol backbone that is made up of a three-carbon molecule that serves as the foundation.Then there is two fatty acid tails one unsaturated and the other saturated, and they are no polar and hydrophobic. Then the Phosphate group is attached to the third carbon of the glycerol. This group is negatively charged making it polar and hydrophilic (the phosphate group can be modified by attaching another small, polar molecule, such are choline or serine, which changes the function of the ________)
Their main function is forming cell membranes and in plasma—they arrange into a lipid bilayers where the hydrophobic tails face inward and the hydrophilic heads face outward, creating a selective barrier that control what enters and leaves the cell.
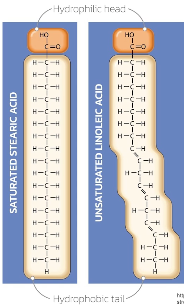
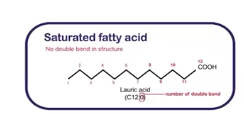
Saturated fatty acids
Some lipids (which are triglycerides and phospholipids) contain fatty acids (building blocks of lipids)
This is a __________ fatty acids and it contains only single bonds between carbon atoms, this makes their hydrocarbon chains straight.
Because the chins are straight, they can pack tightly together (like bricks). This makes the facts soils at room temperature (e.g., butter, lard). So when these are eaten in excess, these tightly packed fats can build up as plaque in arteries, increasing the risk of atherosclerosis, heart attacks, and strokes.
These are mainly found in animal products (butter, cheese, red meat, pork, poultry skin, dairy fats) and some tropical oils (concunt oil, palm oil)
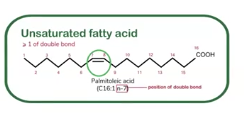
Unsaturated fatty acids
Some lipids (which are triglycerides and phospholipids) contain fatty acids (building blocks of lipids)
This is a __________ fatty acid, and it contain at least one double bond between carbon atoms, this makes kinks or bends in the hydrocarbon chain. The more the double carbon bonds are the more __________ a lipid becomes, making lipids more liquid at room temperature.
Because of the kinks, the chains cannot pack tightly together. This makes unsaturated fats usually liquid at room temperature (like oils)
Since the don’t stack as well, they are less likely to clog artiers, and in moderation, they can even be healthier for the heart by improving cholesterol balance.
These are primarily found in plant oils (such as olive oil, canola oil, and sunflower oil), nuts, seeds, and fish.
Fats
This is a type of lipids and they provide energy storage and support cell function. In some cases, they can also provide insulation to help keep mammals warm, because lipids are poor conductors of heat (because they are nonpolar, so the don’t have charged particles that can move easily, which are needed to conduct heat or electricity, and they don’t transfer vibrational energy efficiently between each other), the _____ layer slows down heat loss from the body to the environment
Steroids
These are a type of lipids that are hormones that support physiological functions including growth and development, energy metabolism, and homeostasis.
A common _____ found in cell membranes is cholesterol, which is essential to structural stability to animal cell membranes (they help maintain the fluidly of the membrane in the animals cells)
Hydroxyl
Is a functional group that has a structure of -OH and it is polar and found in Carbohydrates (sugars), some lipids (glycerol), and nucleic acids.
Carbonly
Is a functional group and its structure is C=O (double bond) it is polar and it is found in carbohydrates (aldehydes and Ketoses).
Carboxyl
It is a functional group and its structure is -COOH it is polar and acidic as it gives up H+ ions, Turing it into COO-.
It is found in Proteins (amino acids), and lipids (fatty acids)
Amino
Is it a functional group and its structure is -NH2 and it is polar/ Basic so it has a tendency to take up H+ ions.
It is found in Proteins (amino acids), and some nucleotides.
Sulfhydrly
It is a functional group and its structure is -SH and it is slightly polar and it is found in Proteins (cysteine) and it forms disulfilde bridges.
Phosphate
Is is a functional group with the structure of -PO4² and it is polar and acidic and it is found in Nucleic acids (DNA, RNA), ATP, and phospholipids.
Methyl
It is a function group and its structure is -CH3 and it is nonpolar and it is found in Lipids, sometimes proteins, and it affects gene expression.
Carbohydrates
____________ are the main file for cells because they can be quickly broken down into glucose, which cells use in cellular respiration to make ATP.
They are made of carbon, hydrogen, and oxygen (1:2:1) and include monosaccharides (like glucose and fructose), and disschardies (sucrose), and polysaccharides (starch, and glycogen).
Carbohydrates have many hydroxly (-OH) groups and glycosidic bonds that enzymes can readily hydrolyze, releasing energy quickly.
Other than energy, they provide structural supports and they sever as recognition/ signalling molecules.
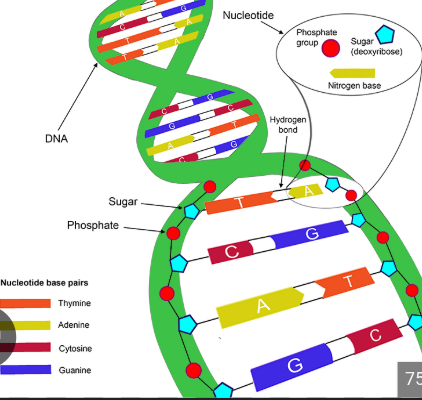
DNA
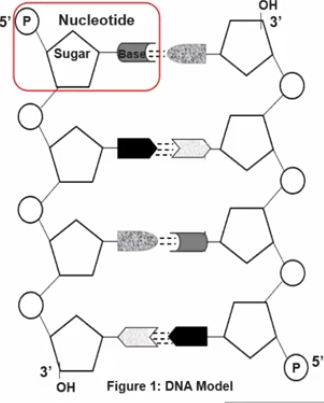
It is a nucleic acids, and it Is a molecule that stores genetic information, which is the instructions needed to build and maintain an organism. It is made up of smaller units called nucleotides.
Each nucleotide consists of three parts; a sugar (carbohydrate), a phosphate group, and a nitrogenous base.
The sequence of these nucleotides determines the genetic code, telling the cell how to make proteins and perform various functions.
Essentially, DNA acts like a recipe book for life, guiding how living things grow and develop.
mRNA
It is a nucleic acids, and it is a type of RNA that carries genetic instructions from DNA to the ribosomes, where proteins are made.
It is produced when a specific segment of DNA is copied during a process called transcription.
_______ is made up of nucletodies, which include sugar (ribose), a phosphate group, and a nitrogenous base.
The sequence of these nucleotides determines the order of amino acids in the protein.
In short, _______ plays a crucial role in synthesizing proteins by conveying information from DNA.
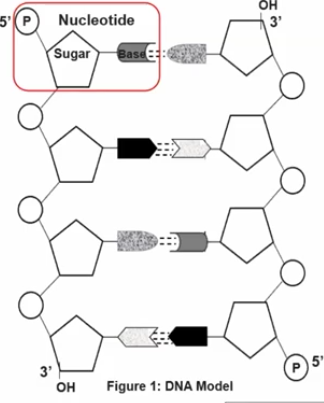
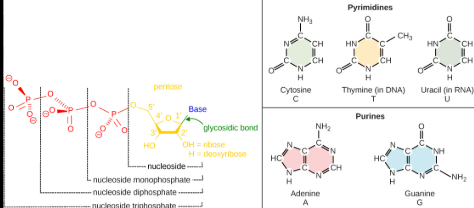
Nucleotides
Is the basic building block of nucleic acids (polymer from _________) like DNA or RNA, not a polymer itself.
These subunits are comprised of a:
5-Carbon sugar (carbohydrate)
A Phosphate group (a central phosphorus atom covalent bonded to four oxygen atoms)
A Nitrogenous base (Is a molecule that contains nitrogen in a ring structure and can either be purines or Pyrimidines)
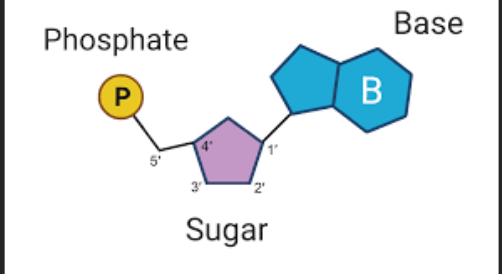
What is each nucleotide monomer connected by.
This is a similarity between DNA and RNA
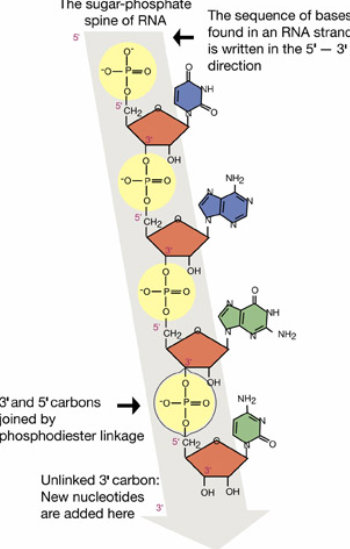
Each nucleotide monomer is connected by covalent bonds forming the sugar-phosphate backbone.
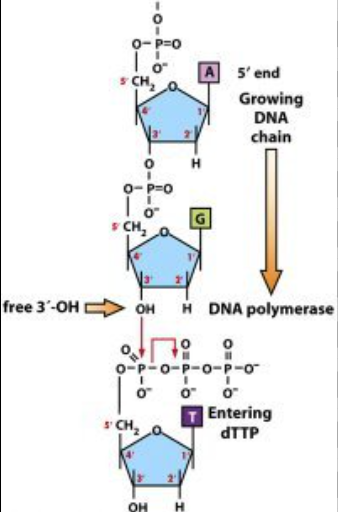
These forms phosphodiesterase bonds, and it is a type of covalent bond that forms the sugar-phosphate backbone of nucleic acids. In this process, the phosphate group of one nucleotide forms a bond with the 3’ carbon of the sugar on the adjacent nucleotide, creating a repeating, strong, and continuous strand.
The linear strand of a nucleotides having a 5’ end and a 3’ end
This is a similarity between DNA and RNA
A nucleotide has a liner strand with two end referred to as the 5’ end and the 3’ end, based on the number of the carbon atoms in the sugar molecule.
The 5’ end has a phosphate group attached to the fifth carbon of the sugar, while the 3’ end has a hydroxyl group (-OH) attached to the third carbon.
They have a direction where:
5’ ends starts
Where the phosphate group is linked to the 5th carbon
3’ ends finishes
Where the Hydroxyl group (-OH) is attached
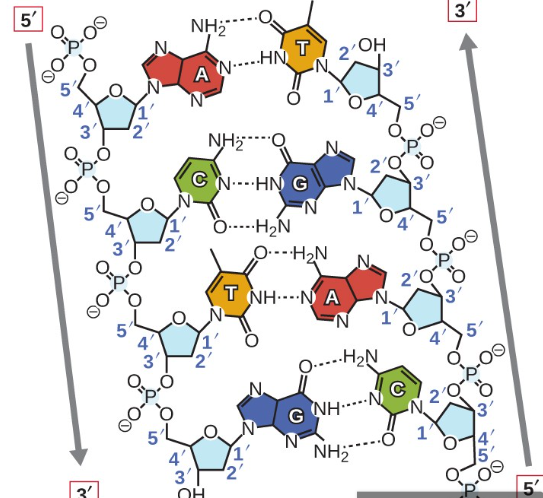
The nitrogenous bases being perpendicular to the sugar phosphate backbone.
This is a similarity between DNA and RNA:
The nitrogenous bases in the nucleotide liner chains (so nucleic acids) are perpendicular to the sugar-phosphate backbone.
As the nitrogenous bases (adenine, thymine, cytosine, guanine, in DNA; and adenine, uracil, cytosine, and guanine in RNA) are attached to the sugar molecules. These bases are oriented perpendicularity to the sugar-phosphate backbone.
This arrangement allows the bases to form hydrogen bonds with complementary bases on opposite strands, as this also helps stabilize the overall structure of the nucleic acids, making it easier for the bases to pair correctly.
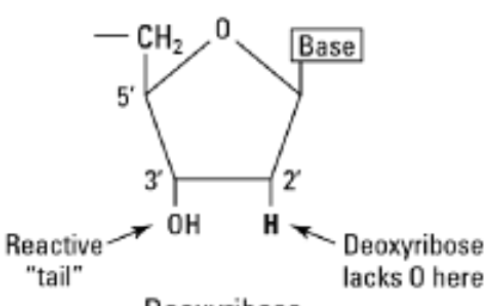
Deoxyribose
Is a difference between DNA and RNA.
This is what DNA contains and it is a five-carbon sugar that is essential to DNA, that along with phosphate groups, forms the molecules backbones and attached to nitrogenous bases.
Its structure is it lacks an oxygen atom found in ribose. This makes DNA more chemically stable and resistant to hydrolysis, which is crucial for storing genetic information over long periods.
The presence of _________ is a key difference from ______ and it contributes to DNA’s double-helical structure, providing stability and a framework for base pairing that encodes genetic information.
Ribose
Is a difference between DNA and RNA.
This is what mRNA contains it is a five-carbon sugar that form a sugar-phosphate backbone, and it is bound to nitrogenous bases.
mRNA contains this to provide a flexible structure for transmitting genetic information. The key functional difference is that _______ sugar as a reactive hydroxyl group on its second carbon, which makes mRNA more susceptible to hydrolysis and less stable than DNA.
This chemical instability is why RNA is suited for its temporary, short-term role as a messenger, whereas DNA is better suited for long-term genetic information storage.
Pryimidines
This are one of two type of nitrogenous bases that make up DNA and RNA, and it is characterized by a single-ring structure.
__________ are one of the two classes of bases that make up the genetic material in nucleic acids.
The three important ___________ bases are Cytosine (C), Thymine (T), and Uracil (U).
Cytosine and Thymine are found in DNA while Cytosine and Uracil are found in mRNA.
DNA’s Pyramidine’s
DNA pyrimidines are nitrogenous bases with a single-ring structure, and these are specifically Cytosine (C) and Thymine (T).
These are essential components of DNA that pair with purines to form the steps of the DNA ladder.
Pyrimidines are one of the two classes of bases that make up the genetic material in nucleic acids.
They Cytosine binds with Guanine purine, and the Thymine binds with the adenine purine. (G-C) & (T-A)
mRNA Pyramidine’s
mRNA pyrimidines are nitrogenous bases with a single-ring structure, and these are specifically Cytosine (C) and uracil (U).
in mRNA, the Adenine (a purine) pars with Uracil (a pyrimidine), than the cytosine (pyrimidine) Paris with the Guanine (a purine)
(A-U) & (C-G)
The complementary base pairing is essential for the correct encoding of genetic information during the process of transcription (where DNA is transcribed into mRNA) and translation (where mRNA is translated into proteins).
The specific paring ensures that genetic code is accurately conveyed, allowing proper synthesis of proteins.
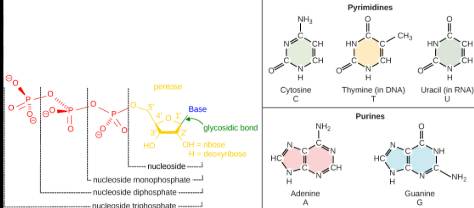
Purinies
Is a two-ringed structure, specifically the nitrogenous bases adenine and guanine, which are components of DNA and mRNA.
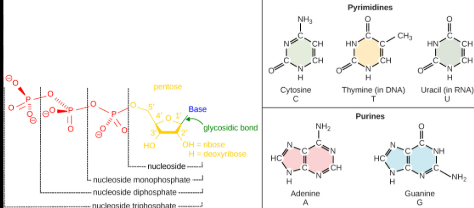
DNA purines
Is one of the two types of nitrogenous bases and DNA’s are adenine (A) and guanine (G)
And they form complementary base pairs with pyrimidines in DNA: adenine always Paris with thymine, and guanine always Paris with cytosine.
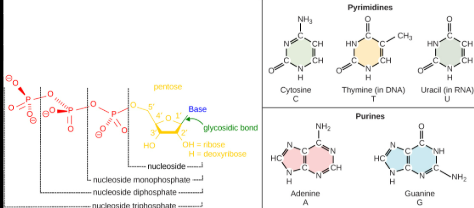
mRNA purines
Is one of the two types of nitrogenous bases and mRNA purines are Adenine (A) and Guanine (G) ; the same as DNA.
And the Adenine (A) pairs with the Uracil (U), and the Guanine (G) Paris with Cytosine (C)
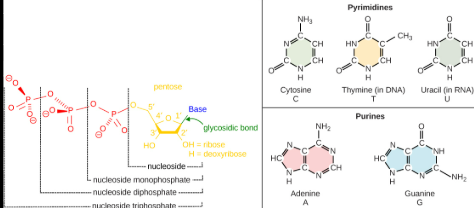
Structure of DNA
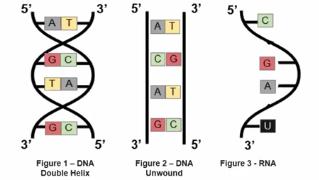
DNA is a double helix means that it consists of two strands that twist around each other, resembling a special staircase.
This structure is caused by the specific paring of the nitrogenous bases (adenine with thymine and cytosine with guanine) and the interactions between the sugar-phosphate backbones of the strands.
The two stands are anti parallel, meaning one runs in the direction from the 5’ end to the 3’ end, while the other runs from the 3’ end to the 5’ end.
This anti parallel arrangement is essential for process like DNA replication and gene expression. (It is a nucleic acids polymer containing two strands, and each strand is an anti parallel 5’-3’ direction)
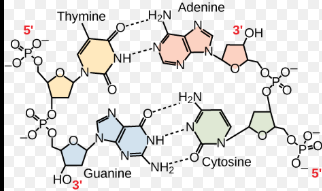
Why is DNA double helix formed
The double helix form of DNA is primarily due to several factors.
First, the specific base pairing between adenine and thymine, and cytosine and guanine creates hydrogen bonds that stabilize the structure.
Additionally, the hydrophobic nature of the nitrogenous bases causes them to orient inward, while the sugar-phosphate backbones faces outward, helping maintain the helical shape.
The twisting of the strands, along with the spatial arrangement of the nucleotides, minimizes steric hindrance and further supports the helical configuration.
Together, these interactions create a stable double helix that is essential for DNA's role in storing and transmitting genetic information.
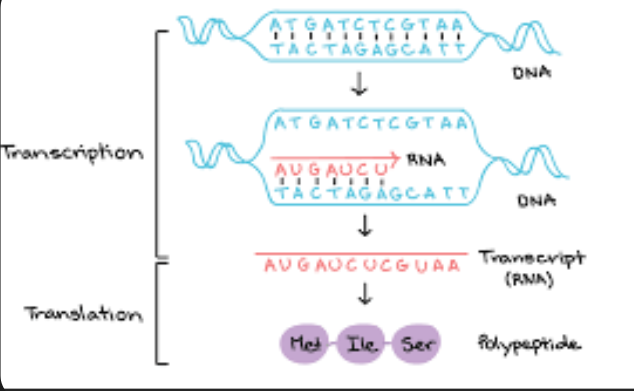
Structure of mRNA being Single Stranded
This is a single-stranded molecule made up of nucleotides, each consisting of a ribose sugar, a phosphate group, and a nitrogenous base.
It is synthesized during transcription, where RNA polymerase reads a DNA template strand and creates a complementary RNA strand.The single-stranded structure of mRNA allows it to easily exit the nucleus and enter the cytoplasm for protein synthesis.
This configuration also enables mRNA to fold into various shapes, facilitating its interaction with ribosomes and other molecules necessary for translation. Overall, the single-stranded form is essential for mRNA's role in transferring genetic information from DNA to protein
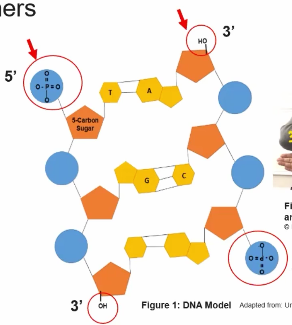
The basic structure differences between DNA and mRNA include
DNA contains Deoxyribose and mRNA contains Ribose
RNA contains uracil (pyrimidines) and DNA contains Thymine (pyrimidines)
DNA is usually double stranded; mRNA is usually Single Stranded
The Two DNA Strands in double-stranded and DNA are Antiparallele directionality.
A hydrogen bonds exist between the pyrimidines and the purines in DNA double Helix structure.
The basic structure similarities between DNA and mRNA include
Both DNA and mRNA had three components—-Sugar, Phosphate group, and a Nitrogenous base— that form nucleotide units that are connected by covalent bonds to form a lineral molecules with 5’ and 3’ ends.
The nitrogenous bases are perpendicular to the sugar-phosphate backbone
The both contain the same Purines groups (Adenine & Guanine) and contain one of the same Pyrimidines groups (Cytosine)
The linear sequence of all nucleic acids is characterized by a 5' end phosphate and a 3’hydroxly of the sugar in the nucleotide.
How would a change in a Nucleic Acids structure (DNA) cause a disruption.
A change in the structure of nucleic acids, such as DNA, can disrupt its function in several ways.
For example, if there is a mutation that alters the sequence of nitrogenous bases, it can lead to incorrect base pairing during DNA replication or transcription.
This may result in the production of faulty proteins or no protein at all, potentially causing genetic disorders or diseases.
Additionally, structural changes, like breaks in the DNA strands or changes in the double helix shape, can affect the DNA's stability and its ability to replicate and repair, further disrupting normal cellular functions.
What bonds exist between pyrimidine and a purine in DNA.
The base pairs, Adenine (purine) and Thymine (pyrimidines) are held together by 2 hydrogen bonds
However, Guanine (purine) and Cytosine (pyrimidines) base Paris are held together by 3 hydrogen bonds.
The more bonds in total between the base pairs in a DNA molecules stabilized the molecules structure.
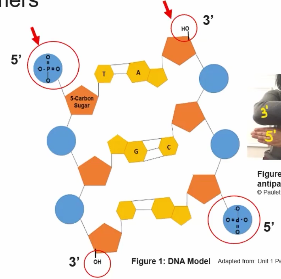
Directionality of the subcomponets influences function of nuclei acid polymers.
The linear sequence of nucleotides encodes biological information, so if any change to the sequence of the nucleotides may lead to a change in the seconded information.
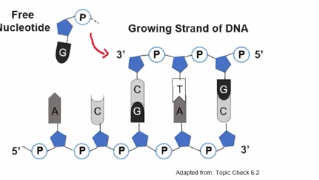
Directionality influences the synthesis of nucleic acids
Ad during the synthesis of nucleic acid polymers, nucleotides can only be added to the 3’ end, as a covalent bond would need to form between the phosphate of the nucletodies and the 3 prime end of that growing strand on that sugar.
Covalent bonds are used to connect free nucleotides to the strand
Deoxyribonucleci acid
What does DNA stand for
Messenger ribonucleic acid
What does mRNA stand for
Proteins
Are one of the most abundant macromolecules (polymers) in organisms, and its subcomponents (monomers) are amino acids.
Once _________ are consumed, they are broken down into amino acids, and they are transported to the cells, where ______ Synthesis occurs in the ribosomes, where amino acids are assembled into ______ based on the instructions in DNA (which are carried by the mRNA).
And the Newly made _______ can go to various locations in the body, including:
Muscles: for growth and repair of muscle tissues
Enzymes: to catalyze biochemical reactions
Hormones: to regulate physiological process
Cell Membranes: To Help maintain structured function of cells
Immune system: To produce antibodies that help fight infections.
Directionality of the subcomponents (amino acids) influences structure of proteins
Proteins comprise linear chains of amino acids that have directionality with an amino terminus and a carboxyl terminus.
Amino acids are connected by the formation of covalent bonds at the carboxyl terminus of the growing peptide chain.
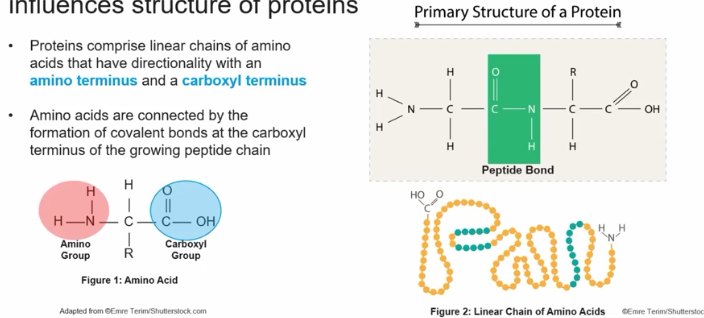
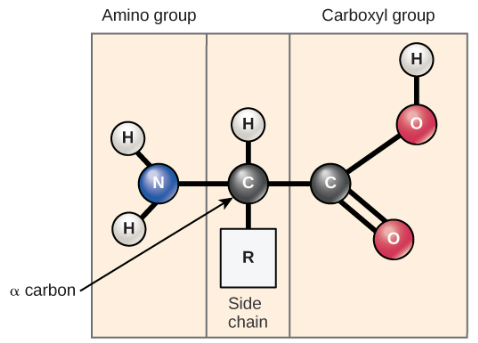
Structure of an amino acid (monomer to proteins)
This monomer makes protein polymers, and it has a Carboxyl group( -COOH) at its terminus and also has an Amino group (-NH2).
If a R group and this gives each molecule its specific properties,.
The Carboxyl group gives this molecule its acidic properties (as it can donate a proton H+ in solution. This ability to releases a hydrogen ion makes this amino acid as an acid)
The Amino group gives it it’s basic properties (as it can take in a H+ proton Turing it into -NH3, so natural to then positive in this circumstance)
Moreover it has a central hydrocarbon.
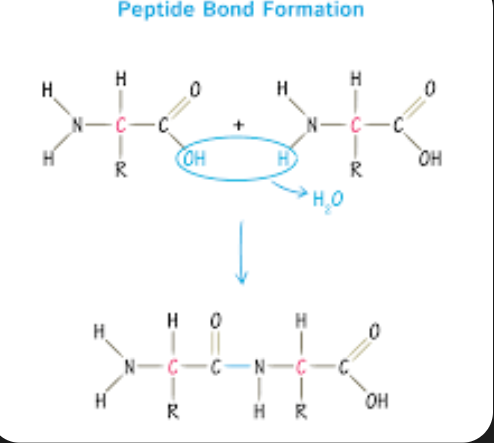
Peptide Bonds
Are the chemical bonds that link amino acids together to from proteins. They occur when the carboxyl group of one amino acid reacts with the amino group of another, releasing a molecule of water in a process called dehydration synthesis.
This bond is crucial for building the primary structure of proteins.
Protein synthesis
Is the process by which cells create proteins using the information encoded in mRNA (messenger RNA). It involved two main steps, transcription and translation.
First transcription occurs in where the DNA is transcribed into mRNA in the nucleus.
Than translation occurs in where the mRNA is translated into a chain of amino acids at the ribosome, where peptide bonds form between the amino acids, linking them together to create a protein.
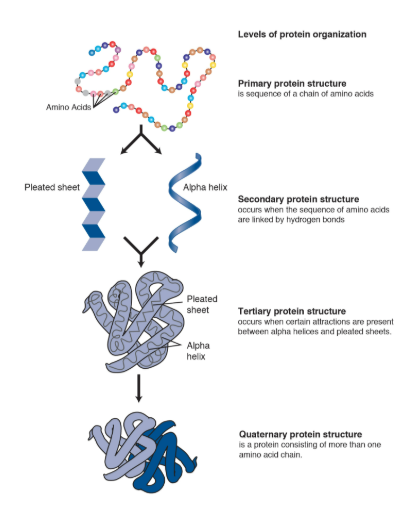
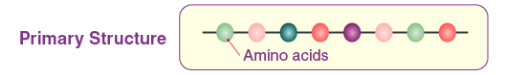
Primary structure
Is the unique sequence/order of amino acids in a polypeptide chain (peptide chain).
This is determined by the sequence order of their constituents amino acids, held together by covalent bonds, called peptide bonds.
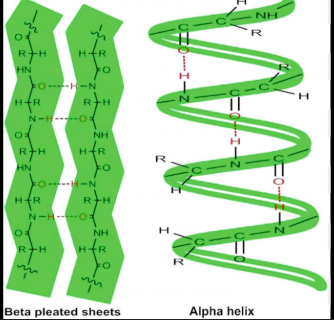
Secondary Structure
The local folding of the polypeptide is some regions. The most common are the a-helix and the B-pleated sheet (often a result of hydrogen bonding).
This is caused by hydrogen bonds forming between the backbone atoms in amino acid sequence. This leads to specific shapes:
Alpha (a) helix: A coiled structure where the polypeptide chain twists into a special, stabilized by hydrogen bonds between every fourth amino acid.
Beta (B) Pleated Sheet: A folded structured where segments of the polypeptides chain lie parallel or anti parallel to each other, forming a sheet-like appearance, also established by hydrogen bonds.
The Hydrogen bonds typically occur between the following function groups (Amine Group -NH2, and the carboxyl group -COOH.
Additionally, hydrogen bonds can also form between side chains (R groups) of amino acids, further contributing to protein structure.
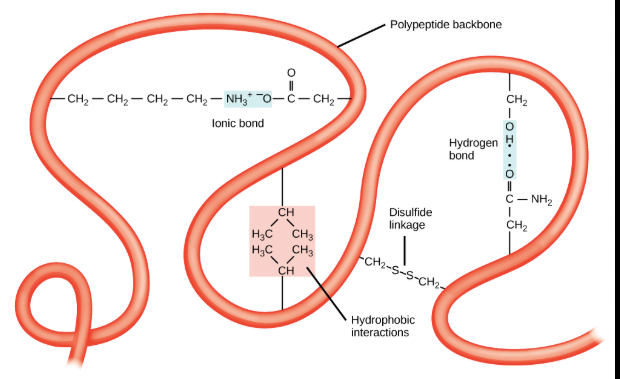
Tertiary Structure
Is the overall 3D shaped of the protein and often minimized free energy; as there is various types of bonds and interactions that stabilized the protein at this level.
Is the Overall 3D shaped of a single polypeptide chain, resulting from the folding of its secondary structures (like alpha-helices and beta-sheets) due to interactions between the amino acid R groups.
Unlike secondary structure, which involves interactions between the peptide backbone, _________ structure is determined by interactions between the “R groups” or side chains of the amino acids.
The type of R groups interactions include:
Hydrophobic interactions: Nonpolar (hydrophobic) R groups cluster together on the inside of the protein, away from the surrounding water, while polar R groups remain exposed.
Hydrogen Bonds: Form between polar R groups, helping to stabilize the protein’s shape.
Ionic Bonds: Attractions between R groups with opposite charges, such as that further stabilize the structure.
Disulfid Bonds: Strong, covalent bonds that form between the sulfur atoms of two cysteine amino acids, creating significant kinks and loops in the polypeptide chain.
Quaternary structure
This is the interactions reasons from multiple polypeptide subunits.
This is the final 3D arrangement of multiple polypeptide chains (called subunits) that associate to form a single, functional protein complex, like hemoglobin which has four subunits.
These subunits, which can be identical or different, are held together by non-covalent interactions such as hydrogen bonds, ionic bonds, and hydrophilic interactions, creating a higher level of functional complexity that is not presents in all proteins.