Chemistry unit 1- section 1: atomic structure
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
Relative atomic mass (Ar)
The average mass of an atom of an element when measured on a. Scale on which the mass of an atom of c-12 is exactly 12
Relative isotopic mass
The mass of an atom of an isotope of an element measured on a scale on which the mass of an atom of c-12
Relative molecular mass (Mr)
The average mass of a molecule when measure on a scale on which the mass of an atom of c-12 is exactly 12
John Dalton-1803
Atoms are spheres and each element is made up of different spheres
1897-J.J Thompson
Discovered the electron
Said atoms wasn’t solid and was made up of other particles
Plum pudding model developed—> positive sphere with negative particles embedded
1909-Ernest Rutherford
Discovered the nucleus which was very small and positive. Concluded the atom was mainly empty space, made up of a negative cloud
Ernest Rutherford- gold leaf experiment
(+) alpha-particles fired at thin sheet of gold leaf.
Most went through the gold leaf = most of atom is empty space
Small number of them deflected back = hit the small (+) nucleus.
1913 Niels Bohr
Discovered a problem with Rutherfords model. The cloud of electrons could collapse into the (+) nucleus but it doesn’t
Why= proposed e- were in fixed energy shells
1913 Niels Bohr
Wondered why the negative cloud doesn’t collapse into the (+) nucleus
Proposed the idea that
e- only exist in fixed orbits(shells)
Each shell has a fixed energy
When an electron moves between shells em radiation is emitted or absorbed
Because energy of shells is fixed, frequency of radiation is also fixed
Atomic model today - Quantum model
Electrons don’t have the same energy shells - we have sub-shells, this explains ionisation trends
Mass spectrometer :
A machine used to analyse elements or compounds
Time of flight mass spectrometer steps
1.) ionisation
2.) acceleration
3.) ion drift
4.) detection
1.) ionistion
First Sample is vaporised so it can move trough TOF mass spectrometer
A.) electrospray ionisation:
Solvent is dissolved and pushed through a small nozzle at high pressure
Then a high voltage is passed through, causing the loss of 1e-
And so a gaseous positively charged sample is produced
B.) electron-impact ionisation:
Sample is vaporised and an ‘electron gun’ is used to fire high energy e- at it
This knocks off an e- off each particle so they become +1 ions
2.) acceleration
(+) ions are passed through an electric field, this gives off the same kinetic energy to all the ions
The lighter ions experience a greater acceleration than the heavier ones despite being given the same k.e
3.) ion drift
Next ions enter a region with no electric field.
They drift through it at the same speed they left the electric field
This means lighter ion drift at higher speeds
4.) detection
Ions detected as the electrical current is made.
When particles hit the plate, those with lower m/z reach the detector first as they travel faster.
Why do we need to need to ionise the sample first ?
To make it accelerate
To deflect/ bend the beam
What is adjusted in the mass spectrometer to enable ions formed to be directed on to the detector ?
The electric field/electromagnet used
When would you use the different types of ionisation in a mass spec?
Electron impact used for organic or inorganic molecules with a low formula mass.
Electrospray used for substances with a higher molecular mass including biological molecules, e.g. proteins.
What are two features of the current model that are not shown in Rutherfords’ ?
Current model includes neutrons and protons in the nucleus
Current model shows electrons in different energy levels/ orbits
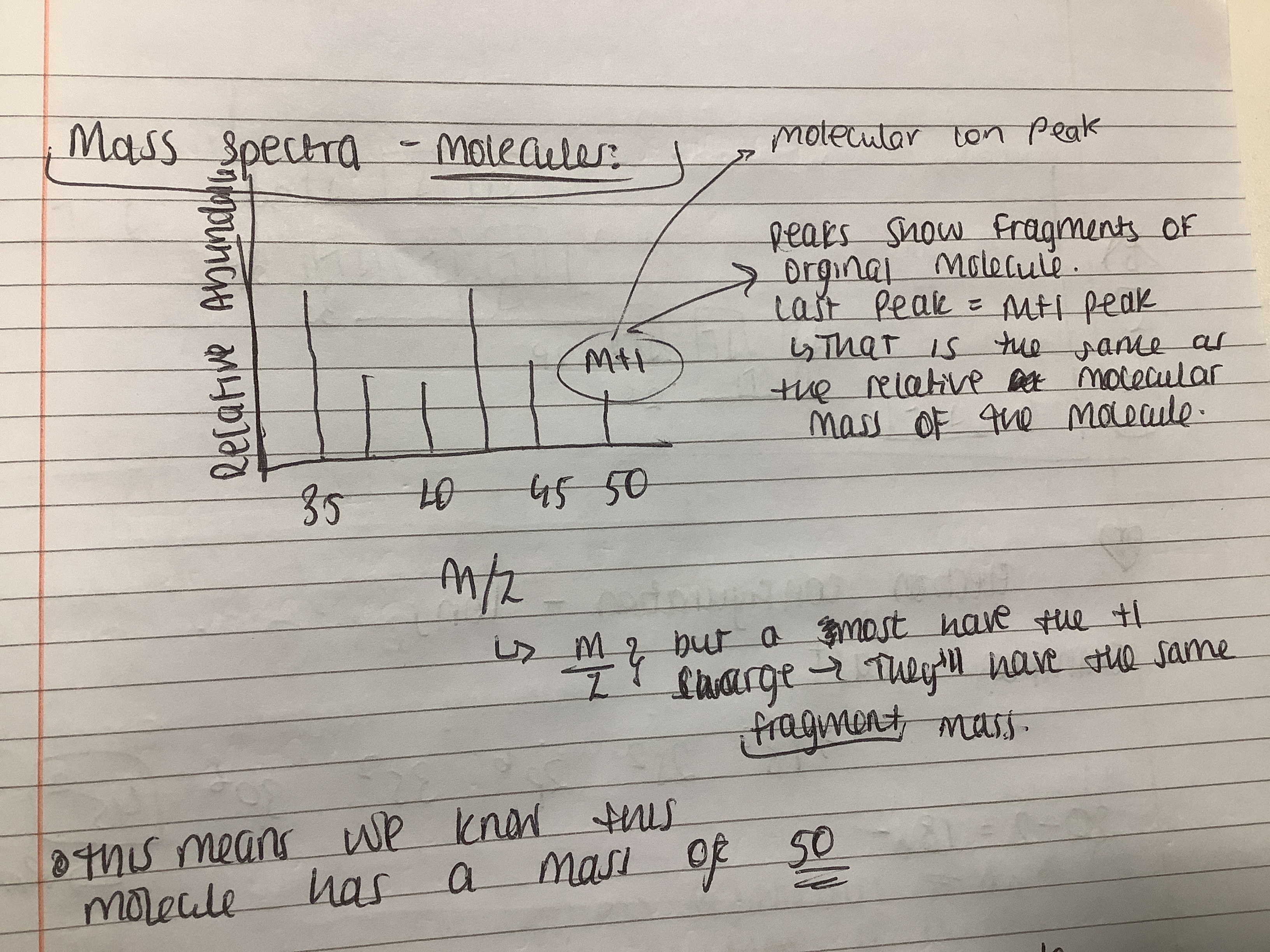
What do the peaks on the mass spectra show?
Peaks show fragments of original molecule
The last peak = m+1, this is the same as the relative molecular mass of the molecule

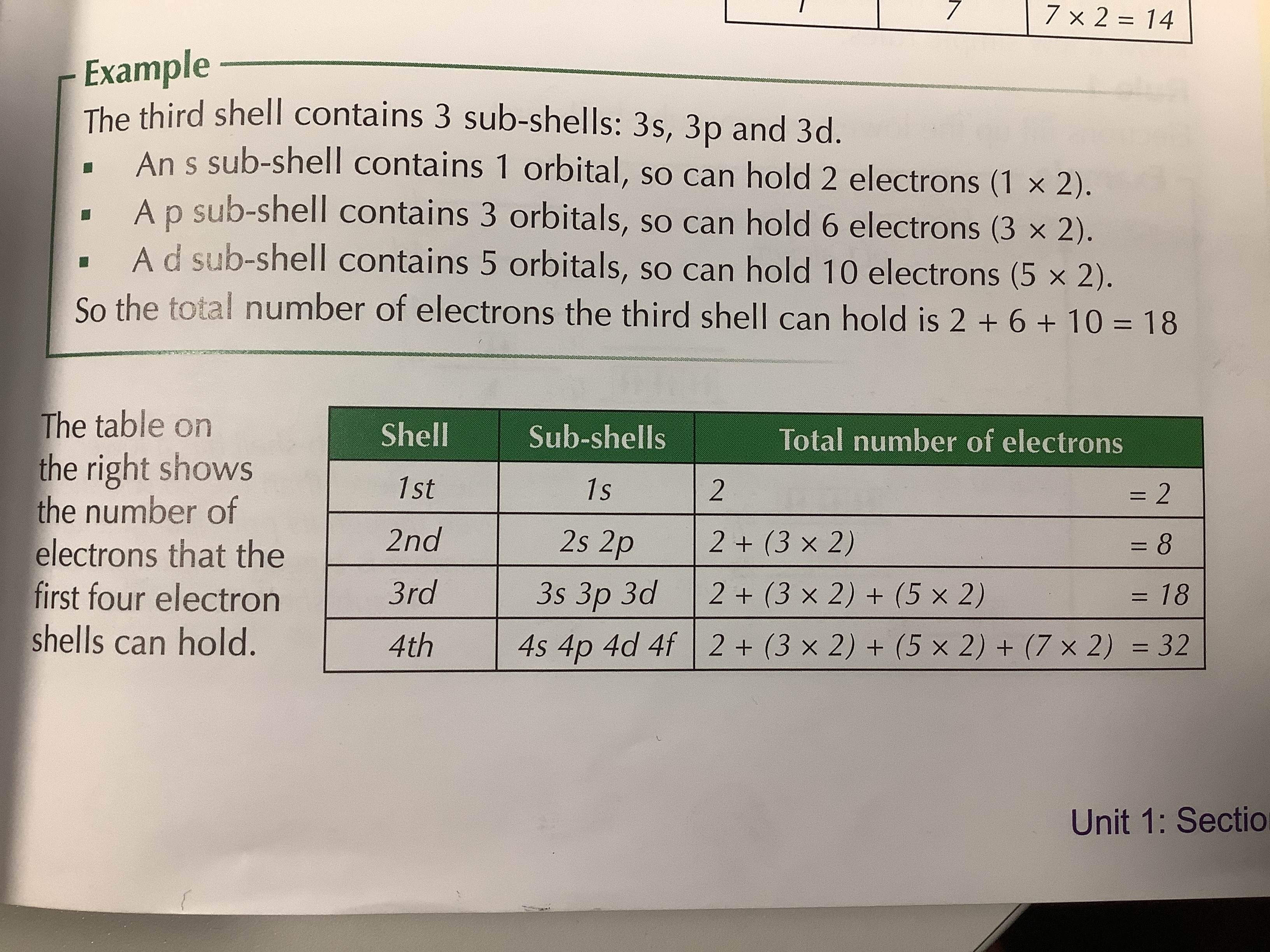
What are the 4 subshells?
S— has 1 orbital and can hold 2e-
P— has 3 orbitals and can hold 6e-
D— has 5 orbitals and can hold 10e-
F— has 7 orbitals and can hold 14e-
Shortened electron configurations
Noble gases in square brackets are sometimes used as short hand in electron configuration
E.g calcium which is 1s2 2s2 2p6 3s2 3p6 4s2 can Be written as
[Ar] 4s2 where [Ar]= 1s2 2s2 2p6 3s2 3p6
Electron configurations of transition metals
Chromium and copper behave differently. For example they donate one of their 4s e- to the 3d sub shell. This is because they prefer to have a more stable full or half- full d-sub shell
e.g) Cr E.C = 1s2 2s2 2p6 3s2 3p6 3d5 4s1 instead of 1s2 2s2 2p6 3s2 3p6 3d4 4s2
Explain why chromium doesn’t fit the trend of E.C
It only has one electron in its 4s orbital before filling the 3d
1s…3p, 4s1, 3d5
Explain why copper doesn’t fit the trend E.C
It only has one electron in its 4s orbital before filling the 3d
1s2……. 3p6 4s1 3d10
What is ionisation energy?
The energy required to remove 1 electron from each atom in 1mol of gaseous atoms to form 1 mol of gaseous 1+ ions.
What is the general formula for ionisation energy
X(n-1) (g) ———>X(n) (g) + e-
N= number of ionisations
What are the three factors that affect ionisation
1) nuclear charge
2) distance from nucleus
3) shielding
How does nuclear charge affects I.E?
The more protons there are in the nucleus, the more positively charged the nucleus is.
This means the electrostatic forces of attraction between the nucleus and the outer electrons are stronger.
So more energy is needed to break these bonds .
How does the distance from the nucleus affect the I.E?
As distance increases the electrostatic forces of attraction decreases.
An electron close to the nucleus will be more strongly attached than one further away.
This means if it ids further away less I.E is needed to remove the electron
How does shielding affect the I.E?
As the number of electrons between the outer e- and the nucleus increases, the outer electron feels less attraction to the nucleus.