Chem 101 Exam 1
1/116
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
117 Terms
chemistry
the study of the structure, composition, and properties of matter
empirical scientific knowledge
based on observations and experiments
hypothesis
a tenative interpretation or explanation of observations and is falsifiable (results may support a hypothesis or prove it wrong)
experiment
highly controlled procedures designed to generate observations that confirm or refute a hypothesis
scientific law
a brief statement that describes the behavior of nature and predicts future behavior; doesn’t try to explain the why or how
law of conservation of mass - Lavoisier
in a chemical reaction, matter is not created or destroyed
scientific theory
a model for how and why nature behaves the way it does; can be validated by experiments, but never conclusively proven
atoms
submicroscopic particles that are the building blocks of ordinary matter
matter
anything that occupies space, has measurable mass, and is made up of extremely small particles
molecules
atoms bound together in specific geometric arrangements
sublimation
solid to gas
deposition
gas to solid
molecules
atoms bound together in specific geometric arrangements
crystalline solid
atoms or molecules arranged in patterns with long-range, repeating order
liquid has a…
fixed volume but not fixed shape; assumes shape of container its in
compressible gas
can force the atoms in a smaller space to reduce volume (ex: sitting on an air mattress)
pure substance
made up of only one component and has an invariant composition that doesn’t vary from one sample to another
mixture
composed of 2 or more components in proportions that can vary from one sample to another
element
single type of atom that can’t be broken in simpler substances (ex: oxygen, carbon, helium)
atomic elements
occur in nature as discrete, individual particles
molecular elements
occur in nature as their most stable form glued together (ex: hydrogen(H2), nitrogen(N2), oxygen(O2)
diatomic elements
occur in nature as pairs of two (ex: bromine occurs as Br2)
compounds
substances of two or more in fixed, definite proportions
homogeneous mixtures/solutions
uniform distribution and composition and properties in all regions of the mixture (ex: wet sand)
heterogeneous mixture
have a non-uniform composition varying from one region of the mixture to another; multiple substances whose presence can be seen (ex: chocolate chip cookie dough)
separating heterogeneous mixtures
easier to separate; separation through decanting where you take wet sand and filter water into a separate container
separating homogeneous mixtures
distillation where its boiled so that the more volatile (easily vaporizable) liquid is separated and then recondensed in a condenser and collected in a separate flask
physical changes
changes that alter only the state or appearance, but not the composition (ex: water boiling, cutting, crushing, sugar dissolving)
chemical changes
changes altering the composition of matter; atoms rearrange and transform the original substance into different substances (ex: iron rusting/oxidizing)
physical property
a property that a substance displays without displays without changing its composition (ex: odor, taste, color, density, changes in state of matter)
chemical property
a property a substance displays only by changing its composition via a chemical change; particles change (ex: corrosiveness, acidity, flammability, toxicity)
energy
the capacity to do work, which is the action of a force through a distance; moves in the direction of the force (ex: pushing a box across the floor)
kinetic energy
energy associated with motion (ex: dropping a weight and the resulting acceleration converts potential energy into kinetic)
potential energy
the energy associated w/ position or composition (ex: a weight held above the ground has potential energy due to its position within Earth’s gravitational field)
objects or systems with high potential energy tend to…
be unstable and have a tendency to change in a way that lowers their potential energy
Law of Constant Composition/ Definite Proportions - Joseph Proust
compounds have a constant proportion of elements of elements irrespective of mass; all samples regardless of source have the same proportions of constituent elements
Law of multiple proportions - Dalton
when two elements form two different compounds, the masses of element B that combine with 1g of element A can be expressed as a ratio of small whole numbers (ex: m oxygen to 1g carbon in CO2/ m oxygen to 1g carbon in CO2 = 2.67/1.33 = 2)
Dalton atomic theory
each element is composed of tiny, indestructible particles called atoms
all atoms of a given element have the same mass and other properties distinguishing them from atoms of other elements
atoms combine in simple, whole number ratios to from compounds
atoms of one element do not transmutate into another element via chemical change'; only switch places and always have the same total # of atoms
electron and who discovered it
the electron is a negatively charged, low-mass particle that was discovered by JJ Thomson through cathode ray experiments; the change of an electron was discovered by Millikan’s Oil Drop Experiment
Thomson Plum Pudding Model
believed all the mass of the atom was contained in the electrons that were spread out evenly through a sphere of positive charge
Ernest Rutherford experiemnt
proved the plum pudding model wrong by performing an experiment shooting positively charged alpha particles through a thin sheet of gold foil; expected to go straight through but some of the particles deflected back showing that the electrons can’t be spread evenly throughout the atom
Rutherford Nuclear Theory
most of the atom’s mass and all of its positive change is contained in the nucleus
most of the volume of the atom in empty space, throughout which tiny negatively charged electrons are dispersed
There are as many negatively charged electrons outside the nucleus as there are positively charged protons within the nucleus, so the atom is electrically neutral
neutron and who discovered it
neutrons are particles in the nucleus with similar mass to a proton, but no electrical charge; discovered by James Chadwick to answer why hydrogen (1 proton) is ¼ the mass of helium (2 protons); reason is helium has 2 protons and 2 neutrons while hydrogen has 1 proton and 0 electrons
metals have a tendency to… and non-metals have a tendency to…
loose electrons and form cations and gain electrons and form anions
ionic bond
when oppositely charged ions attract one another by electrostatic forces to form a crystalline lattice of alternating cations and anions
ionic compound
has a metal bonded to a non-metal through ionic bonds; ionic compounds are always charge neutral
formula unit
basic unit of an ionic compound; the smallest, electrically neutral collection of ions
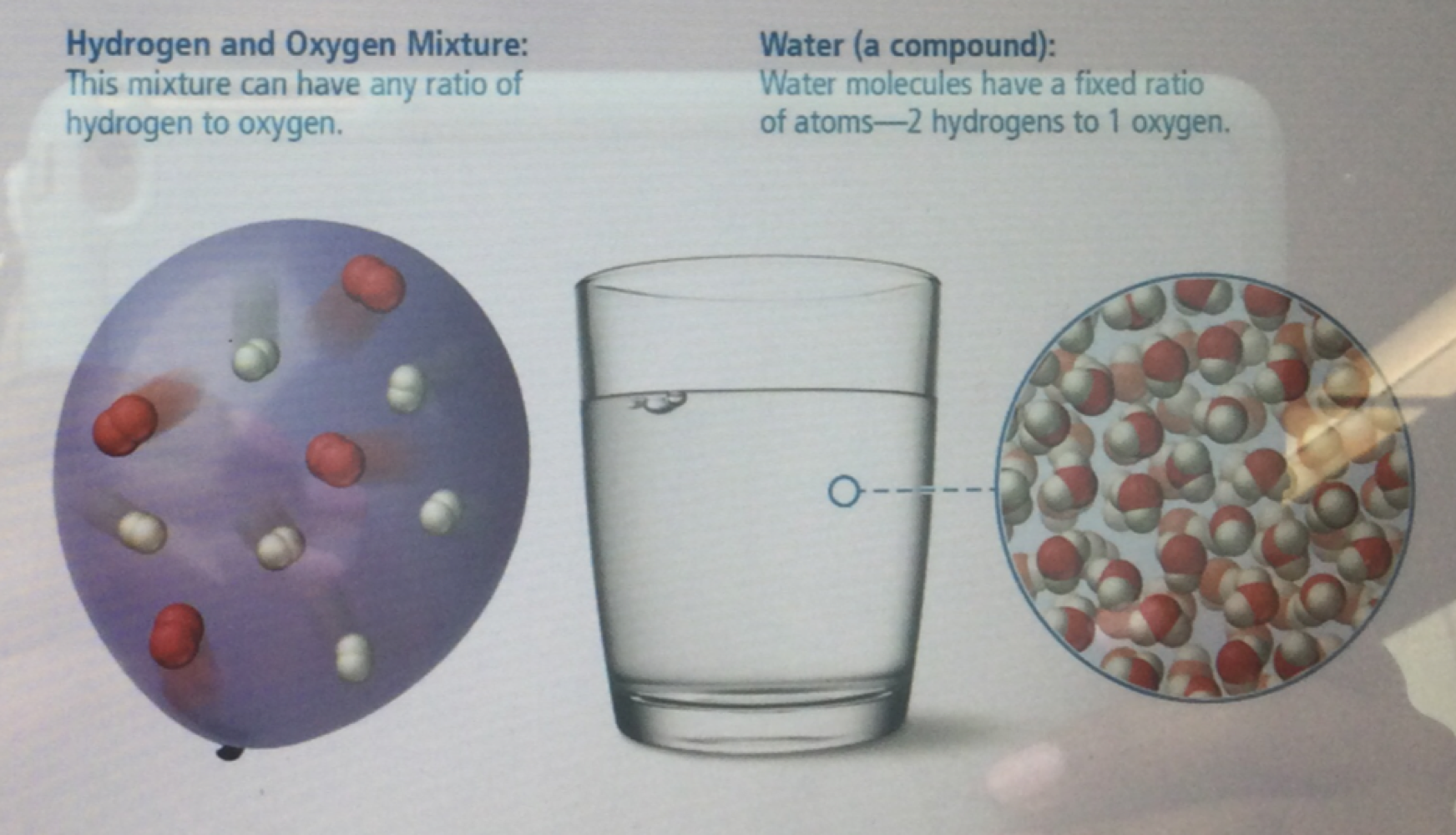
difference between mixtures and compounds
the components of mixtures are not held together by chemical bonds while the atoms of compounds are held together by chemical bonds that form because of the attractions between the protons and electrons of the atoms
molecular compunds
two or more non-metals bonded through covalent bonds; basic unit is a molecule
covalent bonds
two atoms share 1 or more electrons in order to create a molecule with lower potential energy than they do in isolated atoms bc they interact with the nuclei of both atoms
chemical formula
indicates elements present and relative number of atoms or ions; more metallic (+ charged) elements first and less metallic (- charged) elements second
structural formula
uses lines to represent covalent bonds and show how the atoms in a molecule connect or bond to each other
empirical formula
lowest whole number ratio of atoms in an element (HO)
molecular formula
the actual number of atoms in an element (ex: H2O2)
polyatomic molecules
electrically neutral groups or 3 or more atoms held together by covalent bonds (ex: NH4+)
monoatomic molecules
consisting of 1 atom
SI base units (length, mass, time, temperature, amount of substance)
meter (m), kilogram (kg), second (s), kelvin (K), mole (mol)
extensive property
dependent on the amount of a substance (ex: mass and volume)
intensive property
independent of the amount of substance (ex: boiling point)
temperature conversion equations
F = 1.8C + 32
C = (F - 32)/1.8
K = C + 273.15
tera (T)
10^12
giga (G)
10^9
mega (M)
10^6
kilo (k)
10³
deci (d)
(10^-1)
centi ( c )
10^-2
milli (m)
10^-3
micro (u)
10^-6
nano (n)
10^-9
pico (p)
10^-12
1 liter (L) =
1000mL = 1000 cm³
accuracy
closeness to actual
precision
reproducibility; will this produce the same number over and over again
atomic mass
average mass of all isotopes of an element
isotopes
atoms with the same number of protons but a different # of neutrons
atomic number
the number of protons in an atom
mass number
number of protons + number of neutrons
2 isotope notations
mass #/atomic# X (chem.symbol) ; X - mass #
natural abundance
measure of the the average amount of a given isotope on Earth (found by dividing signal intensity of the isotope by the total signal intensity)
ions
atoms with a net charge due to gaining or loosing electrons
cations
positively charged ions from loosing electron(s)
anions
negatively charged ions from gaining electron(s)
1A group charge
+1 cations
2A group charge
+2 cation
3A group charge
+3 cations
5A group charge
-3 anions
group 6A charge
-2 anions
group 7A charge
-1 anions
molar mass
mass of one mole of an atom; numerically equivalent to atomic mass/weight
molecular weight/formula mass
sum of average atomic masses of all atoms in a compound
mass spectrometry
separates isotopes of an element based on mass to charge ratio
metals whose charge is invariant from one compound to another
group 1A and 2A (except for first and last of the groups), scandium, aluminum, zinc, silver (Ag)
metals whose charge varies from one compound to another
transition metals, tin (sn), lead (pb)
nitrate
NO3 -
carbonate
CO3 2-
sulfate
SO4 2-
phosphate
PO4 3-
chlorate
ClO3 -
acetate
C2H3O2 -
Fe
iron