Organic Chem 1 Exam:
1/61
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
62 Terms
1-Carbon chain
Methyl
2-Carbon chain
Ethyl
3-Carbon chain
Propane
4-Carbon chain
Butane
5-Carbon chain
Pentane
6-Carbon chain
Hexane
7-Carbon chain
Heptane
8-Carbon chain
Octane
9-Carbon chain
Nonane
10-Carbon chain
Decane
11-Carbon chain
Undecane
12-Carbon chain
Dodecane
H/CH3 Eclipse Torsional strain
1.3 kcal/mole
H/H Eclipse Torsional strain
1.0 kcal/mole
CH3/CH3 Torsional Strain
3.0 kcal/mole
3-Carbon Ring
Cyclopropane
4-Carbon Ring
Cyclobutane
5-Carbon Ring
Cyclopentane
6-Carbon Ring
Cyclohexane
7-Carbon Ring
Cycloheptane
8-carbon Ring
Cyclooctane
9-Carbon Ring
Cyclononae
10-Carbon Ring
Cyclodecane
11-Carbon RIng
Cycloundecane
12-Carbon Ring
Cyclododecane
Equillibrium favors what in acid/base reaction?
The weaker acid
Strong Acid
Gives up H+ completely in water
Weak acid
Only partially gives up H+
Strong Base
Strongly grabs H+
Weak Base
Weakly grabs H+
Trans
Substituents on opposite sides of the molecule
Cis
Substituents on the same side of a molecule
Confromations
Different shapes of a molecule from bond rotations
Staggered
Lower energy conformations
Eclipsed
Higher energy conformation
Gauche
Steric strain only
Steric strain
Energy increase due to atoms being too close together
Torsional strain
Energy increase due to eclipsed bonds
Bronsted Lowry Acid
Proton donor(H+)
Bronsted Lowry Base
PRoton acceptor(H+)
Strongest intermolecular force
Ion-Ion
Weakest intermolecular force
London dispersion forces
When electrons can go 3 directions what hybridization is a molecule?
sp2
When electrons can go 2 directions what hybridization is a molecule?
sp
When electrons can go 4 directions what hybridization is a molecule?
sp3
What bond angle does an sp molecule have?
Linear 180o
What bond angle does an sp2 molecule have?
120o
What bond angle does an sp3 molecule have?
109o
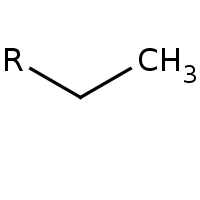
Alkane
No other functional group present
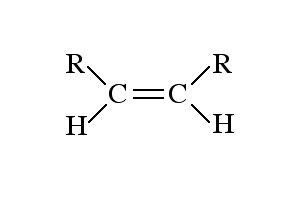
Alkene
C=C double bond
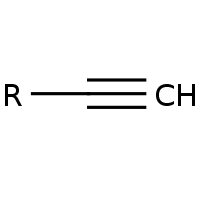
Alkyne
C≡R triple bond
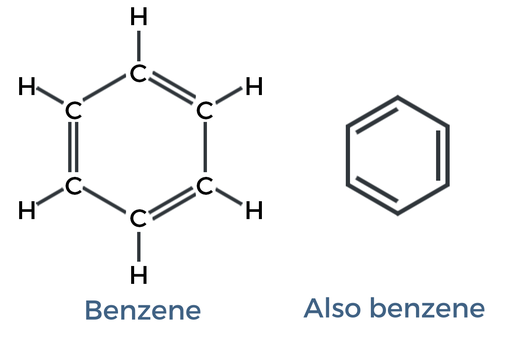
Aromating ring
Ring with alternating double and single bonds
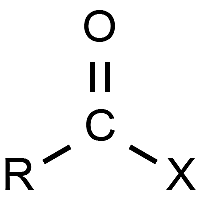
Halide
C-F, C-Cl, C-Br, or C-I bond)
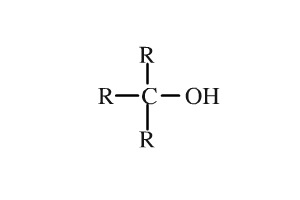
Alcohol
R-OH bond

Ether
R-O-R bond
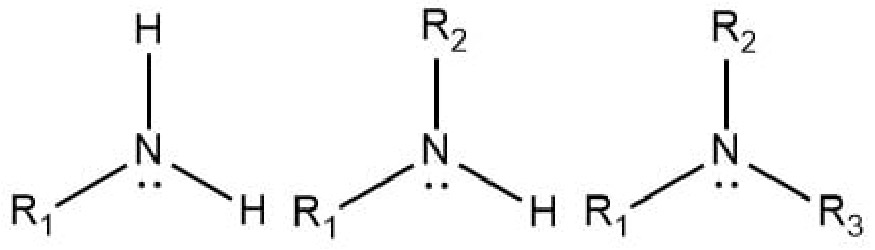
Amine
R-N bond
What determines the degree of an amine?
How many carbons are attached to nitrogen
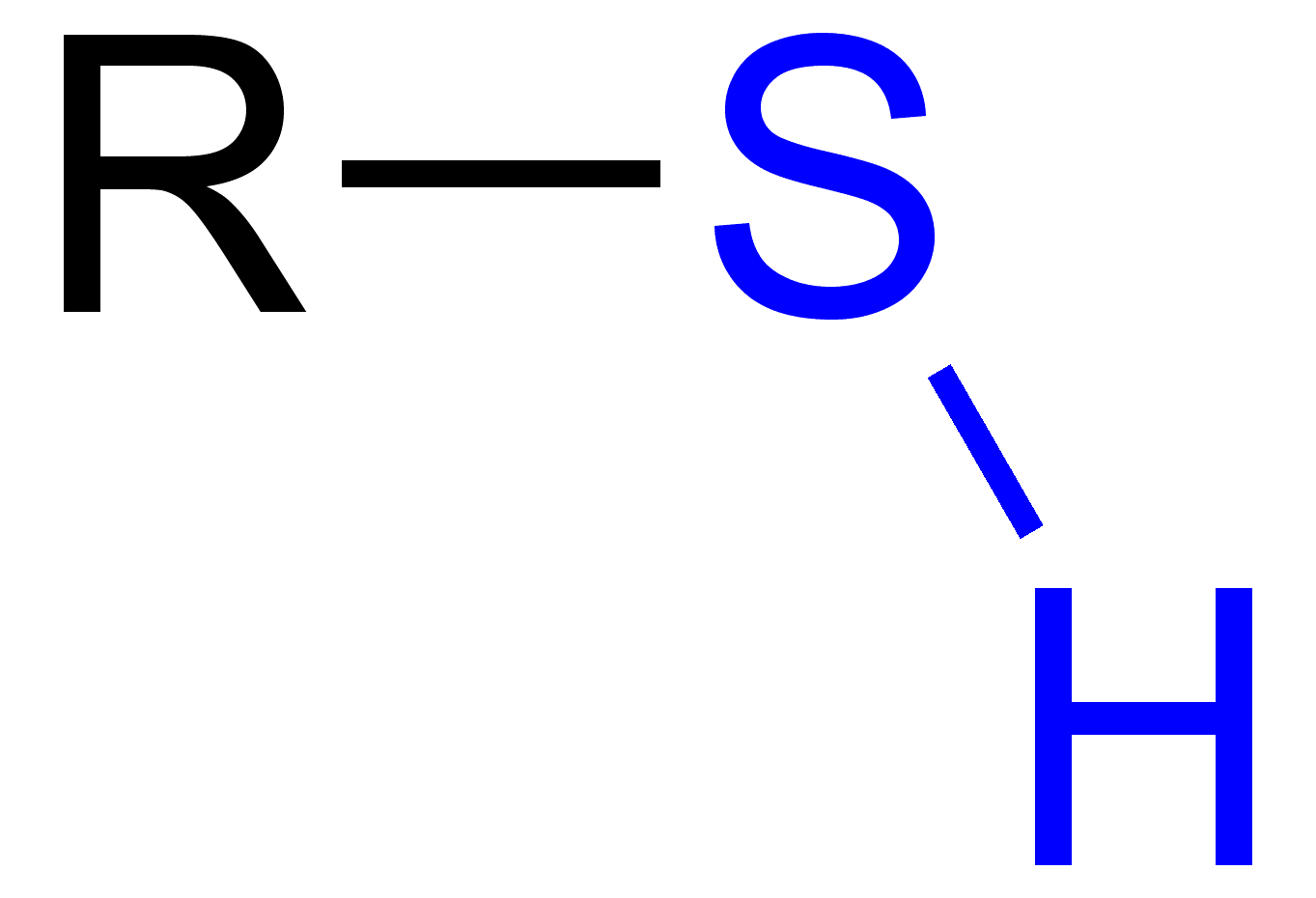
Thiol
R-SH bond
Lewis Acid
Electron pair acceptor
Lewis Base
Electron pair donator
Nucleophile
Lewis Base
Electrophile
Lewis Acid