2.2.1 Electron Structure
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
how are electrons arranged?
orbit the nucleus at different energy levels called shells
what is a shell?
a group of atomic orbitals which have the same principle quantum number, can be split up into separate subshells: s,p,d,f
what is an orbital?
a region around the nucleus that can hold up two electrons with opposite spins
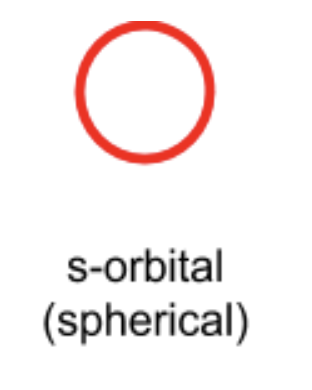
what shape is an s orbital?
spherical
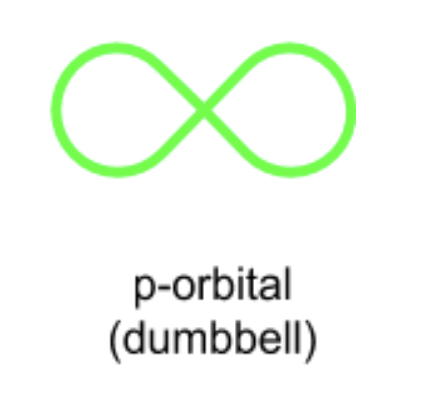
what shape is a p orbital?
dumbell
what is the general formula for the number of electrons in each sub-shell?
2n2
how many s orbitals make up the s sub-shell?
1
how many electrons can an s subshell hold?
2 x 1 = 2 electrons
how many p orbitals make up the p sub-shell?
3
how many electrons can a p subshell hold?
2 x 3 = 6 electrons
how many d orbitals make up the d sub-shell?
5
how many electrons can a d subshell hold?
2 x 5 = 10 electrons
how many f orbitals make up the f sub-shell?
7
how many electrons can an f subshell hold?
2 x 7 = 14 electrons
what are the rules in which electrons fill shells? (3)
lowest energy orbital is filled first,
electrons occupy orbitals singly before pairing up,
no single orbital holds more than 2 electrons
how to electrons pair up?
with opposite spins so atom is as stable as possible, represented by opposite arrows ↑↓
how to electrons fill orbitals?
electrons start from shells with the lowest energy
exception = 4s fills before 3d as it has a lower energy level
for orbitals with the same energy, occupation singly before pairing
what do blocks of the periodic table correspond to?
what orbital the highest energy electron is in