Unit 1: Chemistry of Life
1/22
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
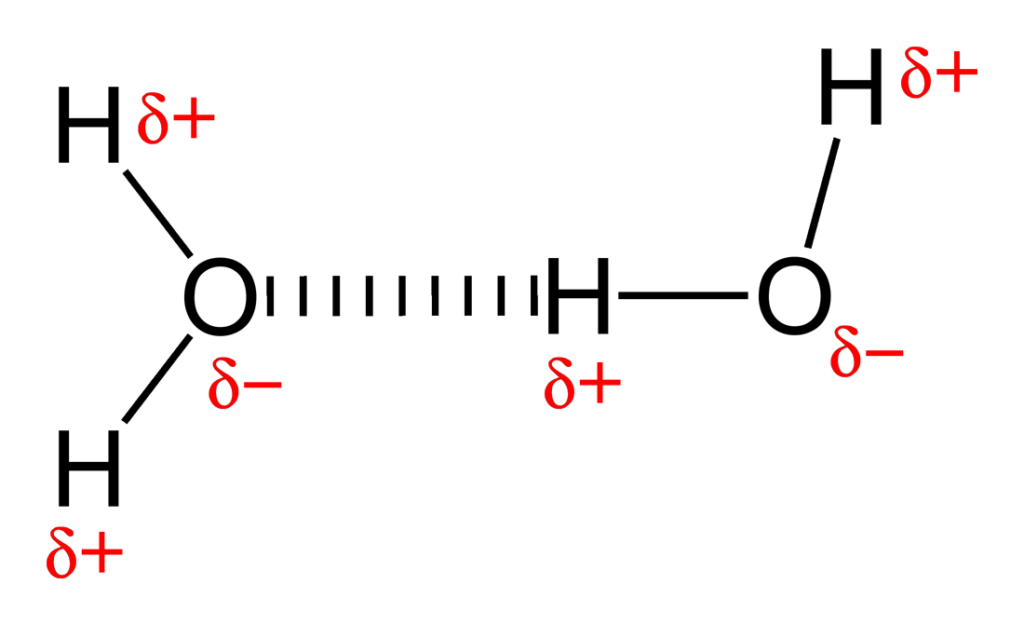
What is a hydrogen bond?
A weak bond between a hydrogen atom of one molecule and an electronegative atom (like oxygen) of another
Polar molecule
unequal sharing of electrons, dissolve in water and other polar substances (water)
Nonpolar molecule
equal sharing of electrons, cannot dissolve in water but can in other nonpolar substances (oils, fats)
Property of carbohydrates (elements, monomer, common types, primary function, bond)
Elements: Carbon, Hydrogen, Oxygen
Monomer: Monsaccharides
Common types: monosaccharides (glucose, fructosem, galactose), disaccharides (Lactose), polysaccharides (starch, glycogen, chitin)
Primary function: Short term energy storage and structural support
Bond: Glycosidic linkage
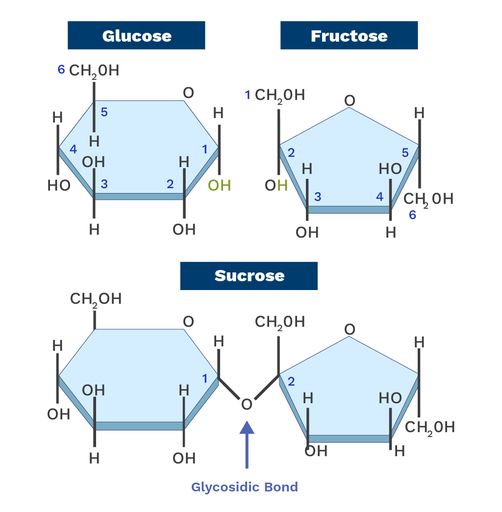
starch vs glycogen
starch: energy storage in plants
Glycogen: energy storage in animals
Both are carbohydrates polyssacharides
-ose
sugar
Properties of Lipids (elements, monomer, common types, primary functions, bond)
Elements: Carbon, hydrogen, oxygen
Monomer: no true monomer
Common types: Fats, Oils, phospholipids, steroids
Primary function: Long-term energy storage, insulation, making cell membranes (phopholipids), and signaling (steroid hormones)
Bond: ester linkage
Glycerol and fatty acids are the "building blocks" of many lipids
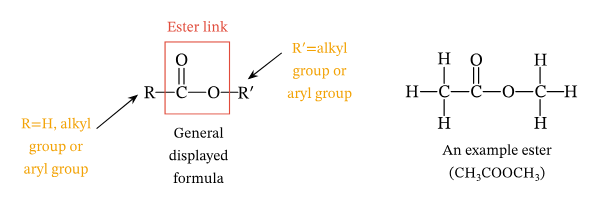
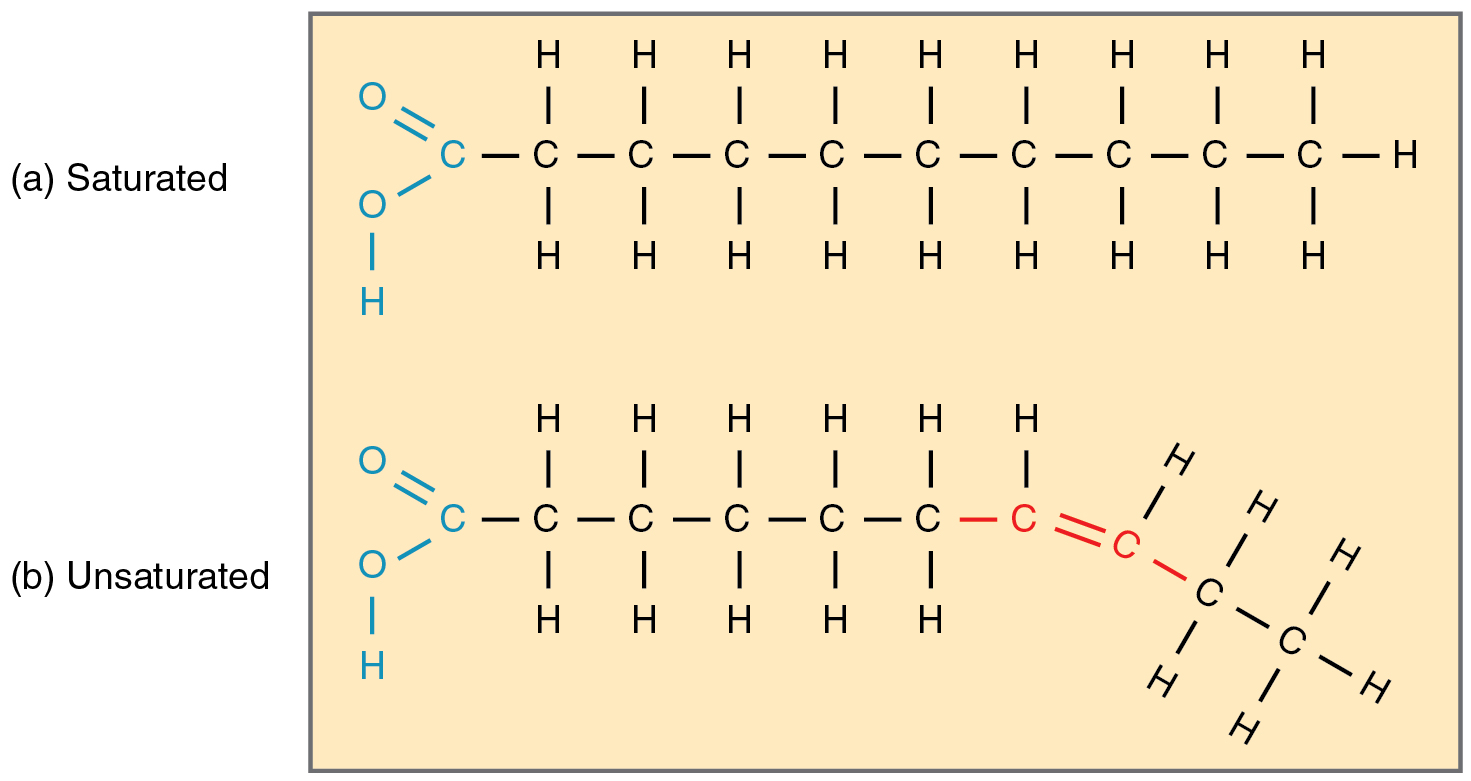
Unsaturated vs saturated lipids (fats)
saturated fats: no double bonds and solid at room temperature
Unsaturated fats: one or more double bonds and liquid at room temperature
Properties of Proteins (elements, monomer, primary functions, bond)
Elements: Carbon, Hydrogen, Oxygen, Nitrogen (sometimes sulfur)
Monomer: amino acids
Primary function: enzymes catalyze reactions, structure (muslce, skin), transport (hemoglobin), communication (hormones), defense (antibodies)
Bond: peptide bonds
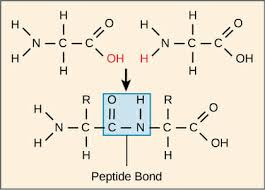
What determines the function of a protein?
Its 3D structure (tertiary structure)
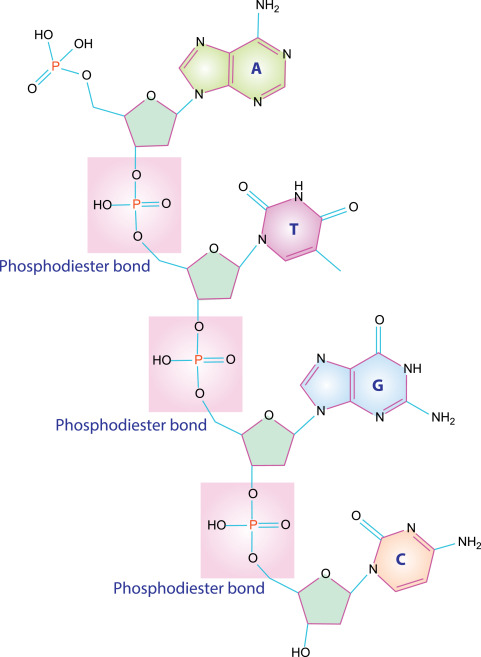
Properties of nucleic acids (elements, monomer, common types, primary functions, bond)
Elements: Carbon, hydrogen, oxygen, nitrogen, phosphorous
Monomer: nucleotides
Common types: DNA, RNA
Primary function: store and transmit genetic information
Bond: phosphodiester bonds (also hydrogen bonds between A-T G-C pairing)
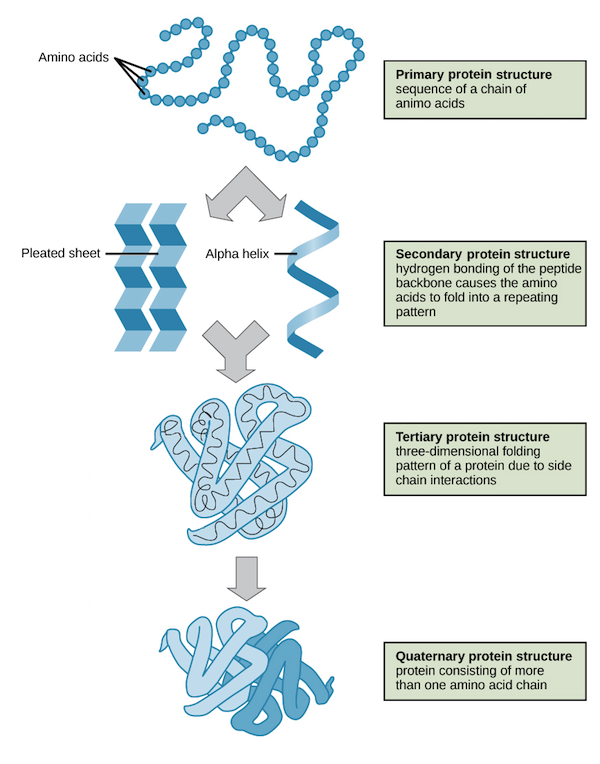
4 structures of proteins
Primary structure: sequence of amino acids
Secondary structure: alpha helices and beta sheets (due to hydrogen bonding)
Tertiary Structure: 3D folding (interactions between r group side chains)
Quaternary Structure: multiple polypeptides combines to form a functional protein compelx
Note: many proteins are fully cuntional after reaching its tertiary structure. Quaternary structures are only needed for proteins made of more than one polypeptide
Function of Enzymes
Speed up chemical reactions by lowering activation energy required for a reaction to occur
Enzymes are very specific!
Structure of Enzymes
Enzymes are mainly proteins
Active site: Region where the substarte binds. Then site is specifically shaped to fit the substrate
Tertiary structure is crucial for proper functioning of the active site
Enzymes sometimes need a little “helper” to help ctalyze reaction so the enzyme may function properly
Cofactors: (usually inorganic - like metals)
Coenzymes (organic - like vitamins)
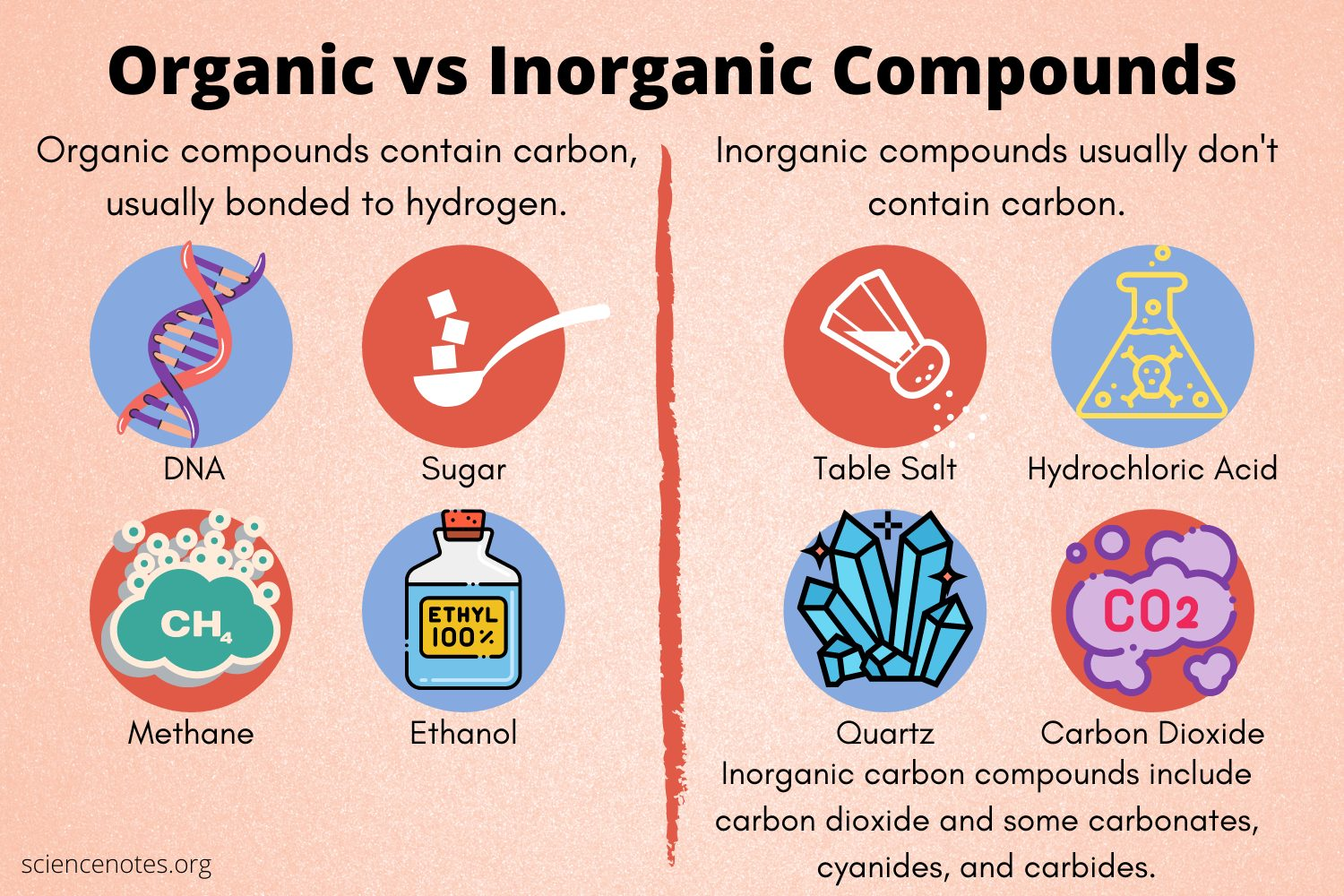
Inorganic vs organic molecules
Ogranic: Carbon + Hydrogen bonded together
Inorganic: No carbon-hydrogen bond
Effects of environment on Enzymes (Temperature, pH, substrate concentration)
Environment:
Low temp: enzymes move slower → slower reactions
High temp: enzymes may denature → lose their shape and stgop functioning
pH
Enzymes have an optimal pH at which they work best
too low or too high → denature
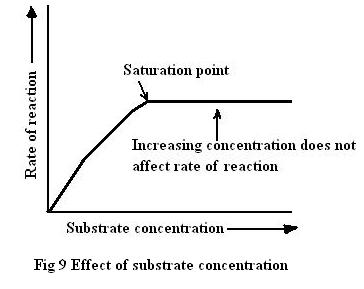
Substrate concentration
increasing substrate concentration → increase rate of reaction (only up to the saturation point where the rate plateaus)
Inhibition of Enzymes
Competitive Inhibition
compete with the substrate to bind to the active site
typically has a simiar shape to the substrate
Noncompetetive inhibition
inhibitors bind to the allosteric site causing a change in the enzymes shape and making the active site less effective
does not directly compete with the substrate
Allosteric regulation
Allosteric activators: activator molecule binds to the allosteric site causing a change in the enzymes shape making the active site more accessible for substrate binding, increasing enzyme activity
Allosteirc inhibitors: inhibitor molecule binds to allosteric site, changes the enzyme shape, and makes the active site less effective at binding the substrate decreasing enzyme activity
Feedback inhibition: the final product of the pathway acts as an inhibitor to the first enzyme in the pathway, preventing it from producing more product.
Negative Feedback Loop: Feedback inhibition prevents the overproduction of a substance by slowing down or stopping its own synthesis when it is no longer needed. It’s an important regulatory mechanism for maintaining homeostasis within cells.
dehydration synthesis
joining two monomers by removing a water molecule
hydrolysis
adding a water molecule to break a bond between monomers
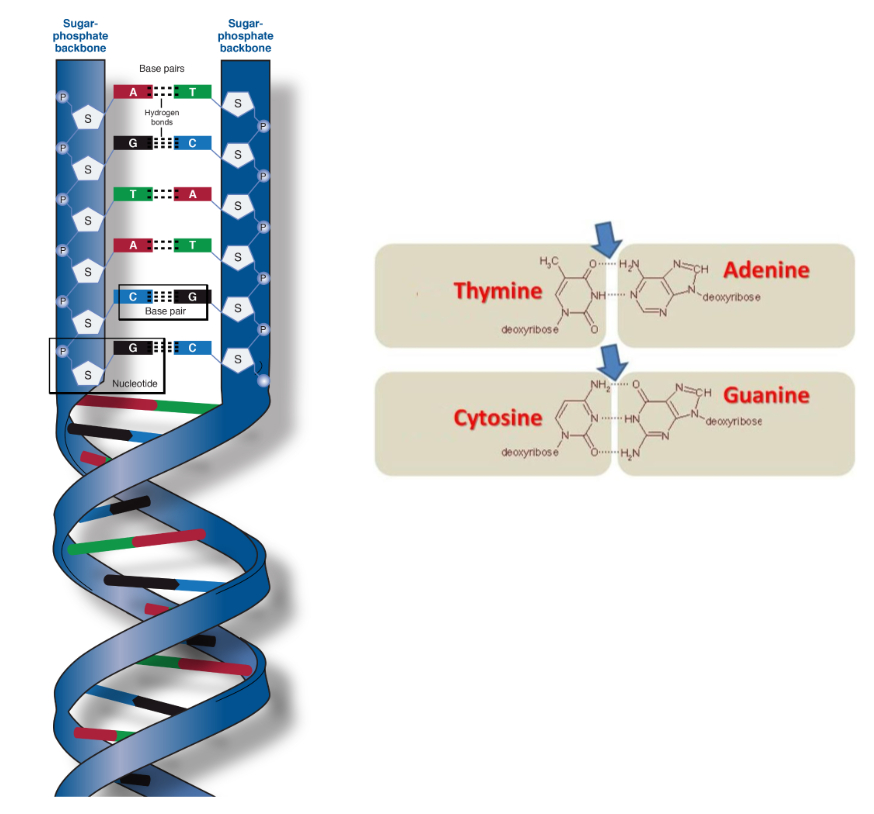
Structure of DNA (shape, sugar, nitrogenous bases, backbone, function)
Double helix
Sugar: Deoxyribose
Nitrogenous bases: Adenine - Thymine (2 hydrogen bonds), Cytosine - Guanine (3 hydrogen bonds)
Backbone: altering sugar and phosphate groups
Function: Stores genetic information (instructions for making proteins)
Structure of rna (shape, sugar, nitrogenous bases, backbone, function)
Single-stranded
Sugar: ribose (one more oxygen than deoxyribose)
Nitrogenous bases: Adenine - Uracil (2 hydrogen bonds), Cytosine - Guanine (3 hydrogen bonds)
Backbone: Sugar-phosphate like DNA
Function: Helps make proteins based on DNA instructions
Elements of life (CHNOPS)
C - central to all oragnic molecules, can form 4 stable bonds, macromolecules
H - hydrogen bonds, water
N - found amino and nucleic acids (DNA, RNA), important in enzymes
O - Crucial for respiration and energy production, found in water
P - phosphate groups in nucleotides (DNA, RNA, ATP), part of energy transfer and storage
S - found in certain amino acids, build disulfide bridges and stablize protein sturcture
These 6 elements are the basic building blocks of life
hemoglobin
protein found in red blood cells that is responsible for carrying oxygen from the lungs to the tissues and organs throughout the body and returning carbon dioxide from the tissues back to the lungs to be exhaled.