protein primary structures
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
how to stabilize an alpha helix
hydrophobic interactions
energetically efficient use of hydrogen bonds
maintain optimal pH and temp
how to destabilize an alpha helix
bulky R groups
get rid of hydrogen bonds
extreme pH, high temp
oppositely charge R groups 3 amino acids away from each other form…
ionic interactions
nonpolar R groups that are 3 amino acids away are close enough in 3D space to form…
hydrophobic interactions
van der Waals interactions can support formation through
bulky side groups (R groups)
Beta sheets
made up of multiple beta strands, can be parallel or anti parallel
Pro B sheets
large R groups
anti-parallel: short distance hydrogen bonding, stronger than parallel
hyrophobic interactions stabilize beta sheet stacking
Anti Beta sheets
repulsion
proline and glycine found in B turns but not sheet itself
Beta turns
4 AA sequence for tight 180degree turn
1st and 4th residues - between carbonyl oxygen and amide hydrogen
proline at position #2
cis confirmation
frequently bent due to bonding of side chain and main chain backbone
glycine at position #3
small side chain allowing it to be small and flexible
Beta sheets in spider silk
the beta sheets of fibroin are stacked on top of each other
side chains are pointing toward and away from you
silk does not stretch because it is in the extended confirmation
flexible, can bend because sheets are held together by non-covalent interactions
globular proteins
made up of alpha-helices, B-sheets, B-turns, and disordered segments
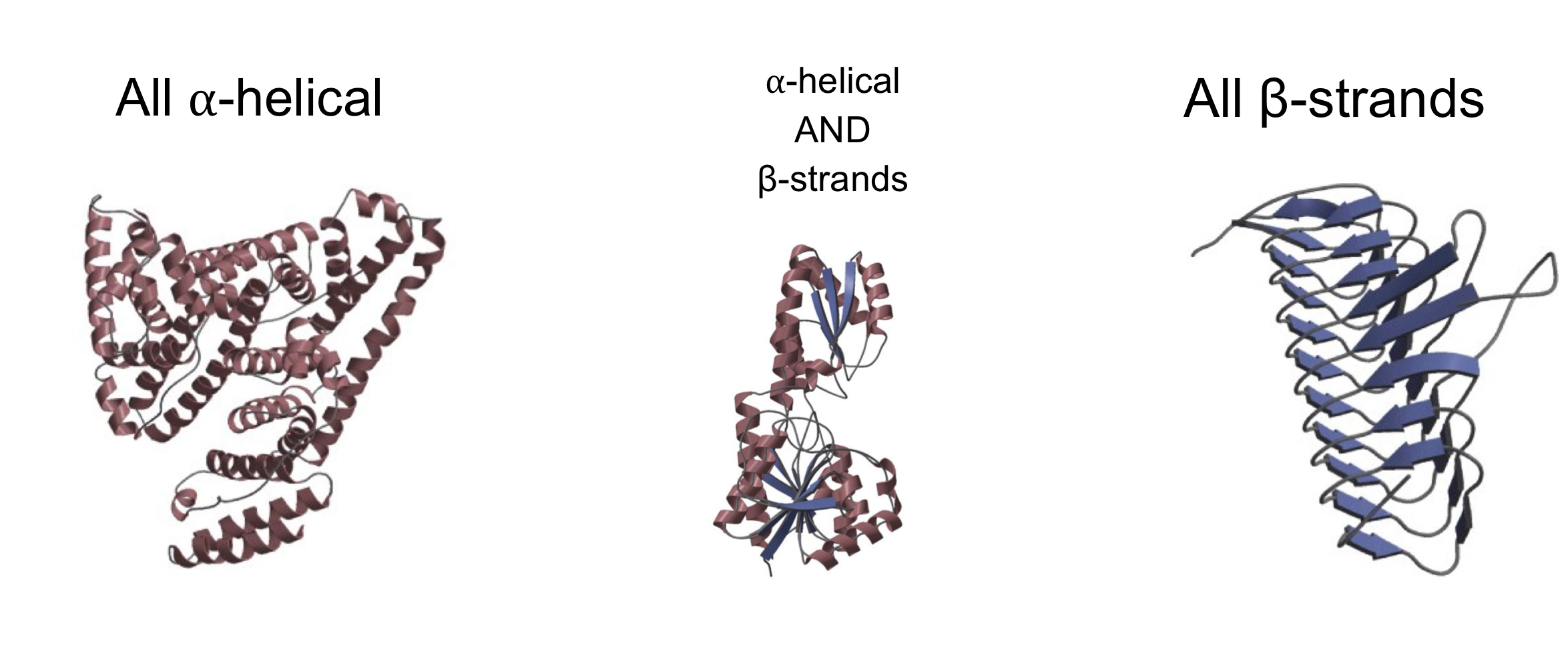
primary protein structure
the sequence of amino acids (from N to C terminus)
secondary structure
describes how a local region of the polypeptide folds (due to interactions between A.A. residues)
different representations of proteins
Ribbon Diagram, Surface Contour, and Space-filling
Common secondary structures form because they optimize _________ and they minimize __________ between atoms!
main-chain hydrogen bonds, steric clashes
Alpha helices
Hydrogen bond every 4th amino acid
R groups oriented externally (since they are bulky!)
Interactions can form to stabilize alpha helix
Energetically efficient use of hydrogen bonds (almost every peptide bond forms a hydrogen bond, each alpha helix turn is supported by 3-4 hydrogen bonds = stable)
ionic interactions
can form between 4th amino acids R groups to stabilize
hydrophobic interactions
can also form between relevant 4th residues to stabilize helix
tertiary structure
overall 3D shape of entire protein (fiber vs globular)
Quaternary structure
overall structure of 2 or more polypeptides interactions
motifs
folding pattern involving two or more types of secondary structure
Domains
part of a polypeptide chain that is independently stable/able to move. Domains can contain motifs
Based on thermodynamics, main idea is that…
folding is favorable! (-ΔG )
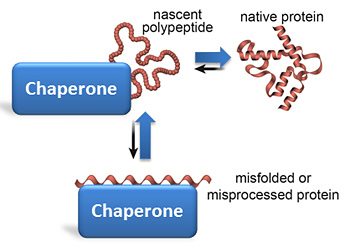
chaperone
a protein that helps other proteins fold correctly
Levinthal’s Paradox
takes way too long to try every combination so there are steps to folding (secondary structure form first)
some proteins need the help of chaperones