Quiz 7 - Intracellular organisms & Viruses
1/122
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
123 Terms
Common features of obligate intracellular organisms:
microorganisms that are incapable of replication outside a living host cell
Cannot be cultured on an artificial medium such as agar plates or broth, even when supplemented with serum or whole blood from the host.
Use animal or human cell cultures most commonly utilized for isolation.
Culture techniques will be examined in more detail in the Virtual Laboratory presentation (material from Ch. 16 in text)
Can not be visualized by the naked eye, or by light microscopy
Microbes are often too small for light microscopy, and cannot form discrete colonies on solid agar to visualize by the naked eye
Organisms may be observed intracellularly; may form inclusion or cause other cellular effects that may be visualized microscopically
Chlamydiaceae Family:
Bacterial intracellular organisms such as those pathogens in diseases with associated intracellular forms which play role in their pathogenicity as well as identification/detection of disease
Rickettsia, Coxiella & other related species:
another Family of organisms which require a host cell to reproduce. Zoonotic agents typically, but also occasionally cause human infections.
Viruses:
These are another group of obligate intracellular
organisms, and are among the smallest, as well as most diverse infectious agents which cause widespread disease worldwide.
DNA Viruses
RNA Viruses
Two Genera of family Chlamydiaceae: Chlamydia and Chlamydophila
Pleomorphic, gram-negative bacteria which only exist as obligate intracellular parasites
Unable to generate their own ATP (energy source)
Infected cells contain intracytoplasmic inclusions around the nucleus of the host cell
Unique Reproductive Cycle of Chlamydiaceae:
Infectious form known as the elementary body (EB)
EB taken into host cell by phagocytosis
EB reorganizes into reticulate body (RB)
RB divides by binary fission then becomes EB once again
Host cell lyses releasing EB to infect other cells
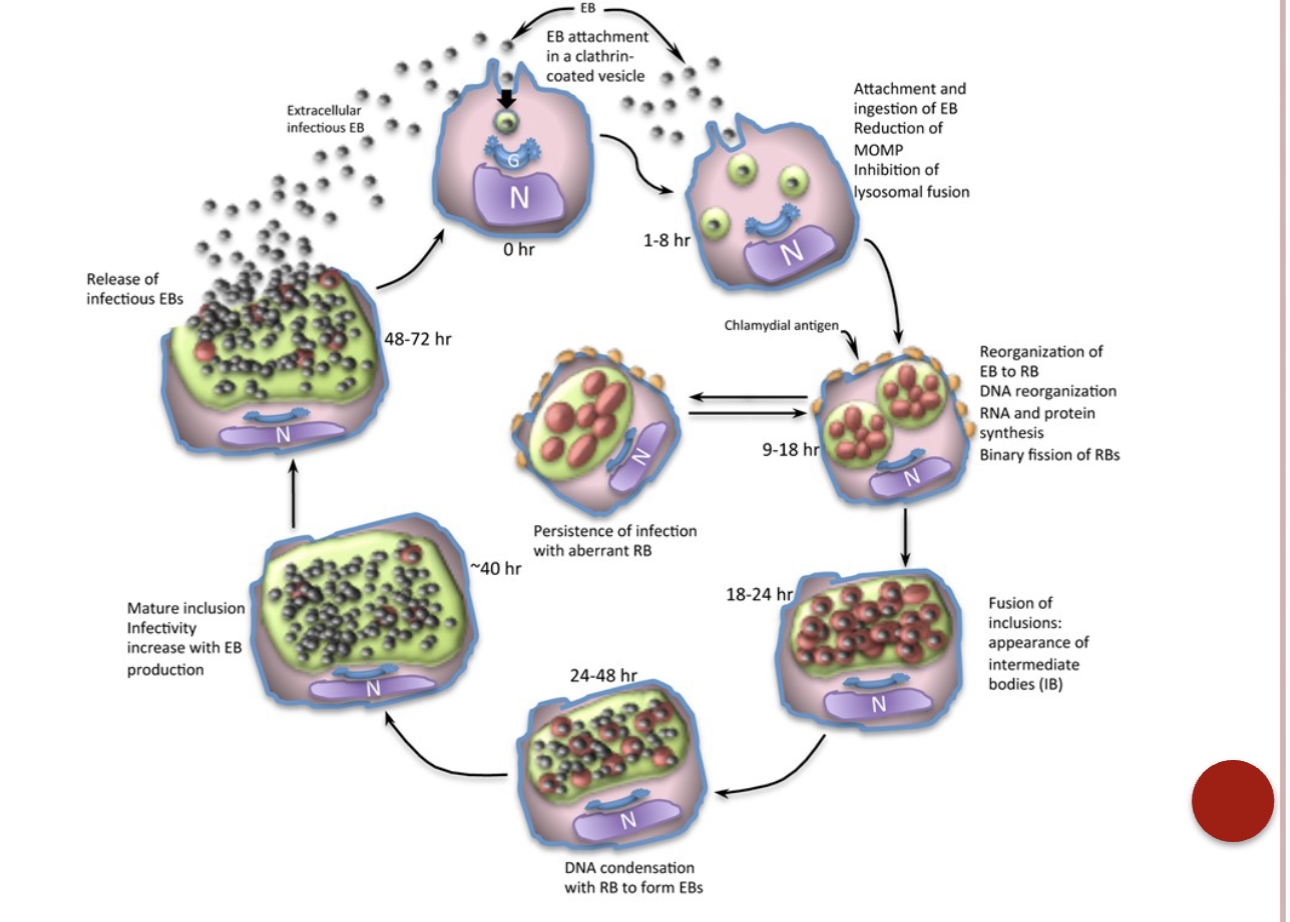
Chalmydia life cycle
Chlamydia trachomatis: etiologic agent of:
Ocular Trachoma
Important cause of blindness due to conjunctival scarring
Occurs worldwide, mainly among poor in areas such as the Middle East, North Africa, northern India, and in Native Americans in the southwestern US. Infections on transmitted from person to person.
Nongonococcal and post-gonococcal urethritis most common sexually transmitted diseases, replace gonorrhea.
Can cause PID, ectopic pregnancy & infertility if left untreated
Organism spreads into fallopian tubes and eventually the into peritoneal cavity causing scarring and pelvic inflammatory disease.
Lymphogranuloma venereum
Serovars L1, L2, L2b, and L3 are invasive in contrast to A-K
Another sexually transmitted disease (STD) found in Africa, Asia, and South America
Causes lesions, draining ulcers, and eventual lymphadenopathy after 2-6 weeks
1st Stage- lesion at the site of inoculation
2nd stage- acute lymphadenitis- enlarged lymph node and forming a large area of groin swelling or bubo.
3rd stage- a chronic stage causing the development of genital hyperplasia, rectal fistulas, draining sinuses, and other manifestations.
Inclusion conjunctivitis
Neonates acquire by passage through the birth canal of infected mothers
Differs from Trachoma infection as there is no corneal scarring.
Adults can also acquire conjunctivitis through hand-to-eye contact with urogenital secretions containing the organism.
List of etiologic agents stemming from Chlamydia trachomatis:
Ocular Trachoma
Non-gonococcal and post-gonococcal urethritis
Lymphogranuloma venereum
Inclusion conjunctivitis
Isolation & Cultivation of Chlamydiaceae:
Direct Microscopic Examination:
Rapid but not sensitive (60% sensitivity)
Fluorescent antibody technique
Greater sensitivity than direct microscopic examination
Fluorescein-labeled monoclonal antibodies attach to elementary body (EB), making them visible with apple-
green fluorescence
This technique can be utilized to detect organisms in
clinical specimens such as conjunctival scrapings,
sputum, throat specimens, pleural fluid, urethral and
cervical scrapings, rectal swabs, and lymph node
aspirates.
Chlamydiaceae culture examination methods:
Cell culture on HeLa, McCoy and monkey kidney cells
for cultivation of organisms
Monolayer of cell culture is stained and examined for perinuclear cytoplasmic inclusions.
Iodine, Giemsa, and immunofluorescent stains can be utilized to visualize the organisms.
Chlamydia trachomatis produces compact inclusions containing glycogen, which stain with the iodine as seen in
Nucleic acid amplification and DNA probe
Increases sensitivity and specificity of detection of organisms
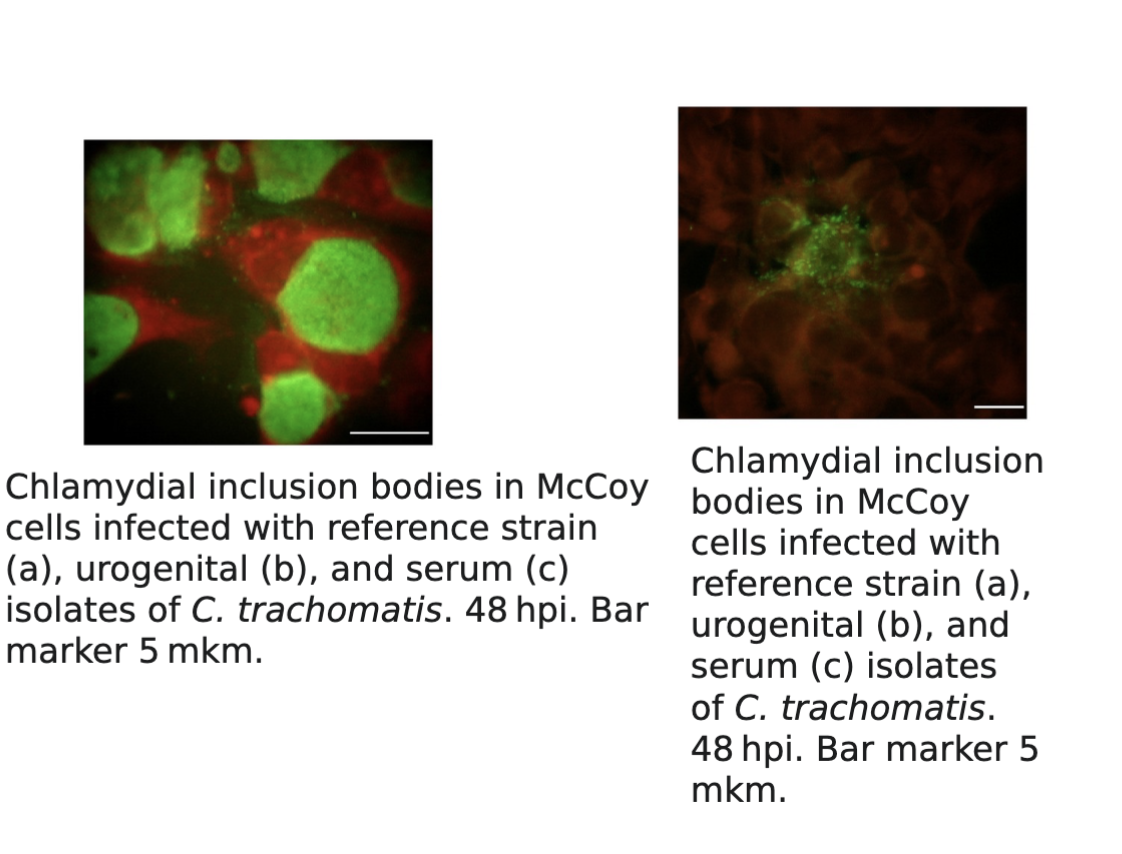
Chlamydia trachomatis detection & treatment:
can be detected by culture, antigenic detection such as immunofluorescence, or nucleic acid assays.
Often individuals are co-infected with Neisseria gonorrhoeae and Chlamydia trachomatis.
Often treated for both organisms and tested for both- STI
Azithromycin and doxycycline are recommended for treatment.
Important note: In cases of sexual abuse and other situations that require results to be used in court of law, Chlamydia trachomatis culture was the gold standard. Nucleic acid amplification tests (NAATs) are now the preferred method due to their higher sensitivity and ability to provide faster results.
Chlamydophilia psittaci: Etiologic agent of psittacosis: General characteristics
also known as parrot fever
primarily an animal disease (birds and other animals) that is transmitted via inhalation as the result of an occupational exposure
Zoo worker, poultry farmer, veterinarian, pet shop worker
Infections range from asymptomatic to mild, to fatal cases of pneumonia
Symptoms include fever, fatigue, chills, headache, and a non-productive cough
Chlamydophilia pneumoniae:
Associated with an acute respiratory illness which occurs worldwide
pneumonia, bronchitis, pharyngitis, sinusitis, flu-like illness, persistent cough
Transmission is person to person (no animal reservoir
Specimen of choice is nasopharynx epithelial cells but not sputum (because there is not really a productive cough)
Linked to atherosclerosis (hardening of the arteries) and coronary heart disease
C. psittaci & C. pneumoniae detection:
can be diagnosed by cell culturing, immunofluorescence or through serological procedures such as complement fixation (CF).
In CF test, EB provides antigen source; infected individuals
demonstrate a four-fold increase in titers.
Other Chlamydia spp can cross-react with complement fixation
C. psittaci & C. pneumoniae treatment:
requires use of tetracycline or doxycycline.
Erythromycin is effective for individuals who cannot be
prescribed tetracycline, such as for children and pregnant
females.
Rickettsia general characteristics:
highly pleomorphic
Coccobacilli, rods, long filaments
Gram negative (stain poorly)
Stain readily with Giemsa
Do not grow in vitro (outside of host cell)
Can be grown in embryonated eggs or tissue culture
Cultivation is costly
Hazardous; easily transmitted by aerosol
Rickettsia: transmission:
by arthropod vectors (except Coxiella)
No person to person transmission
Rickettsia and Orientia spp. are transmitted by the bite of infected ticks or mites or by the feces of infected lice or fleas.
Manifestations of Rickettsia species:

Common clinical manifestations of the Rickettsial Diseases:
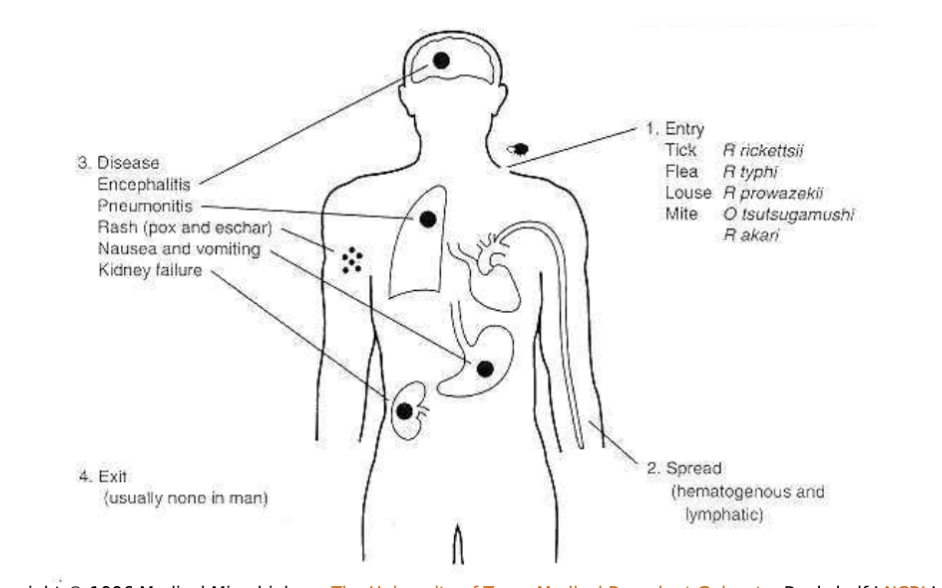
Rickettsia and other related species general characteristics:
Require host cell for reproduction
Most are zoonotic agents
Very small and stain poorly with Gram stain but visualization can be better with Giemsa
Appear gram negative; cell walls containing lipopolysaccharide, lipoprotein, and peptidoglycan.
Very susceptible to environmental changes
All, except Coxiella burnetii, are transmitted from animals by arthropod vectors (ticks, lice and mites)
Infections associated with symptoms such as fever, headache, myalgia, and in some cases a rash, which is seen primarily in rickettsial infections.
Rocky Mountain spotted fever, rickettsialpox, and typhus fevers are all rickettsial illnesses and many occur worldwide
Because its signs and symptoms mimic other infections, accurate testing is necessary for detection.
Rickettsia and Orientia: How it affects an individual:
Enter the endothelial cell of blood vessels
Damage occurs due to lesions throughout the body’s vascular system
R. rickettsii general characteristics:
Rocky Mountain spotted fever (RMSF)
Endemic in U.S. and found primarily in children
Triad of symptoms
Fever, head and rash
Fig 31-5 displays characteristic rash associated with RMSF
History of tick bite during the warm months
Diagnose using cell culture and serological tests
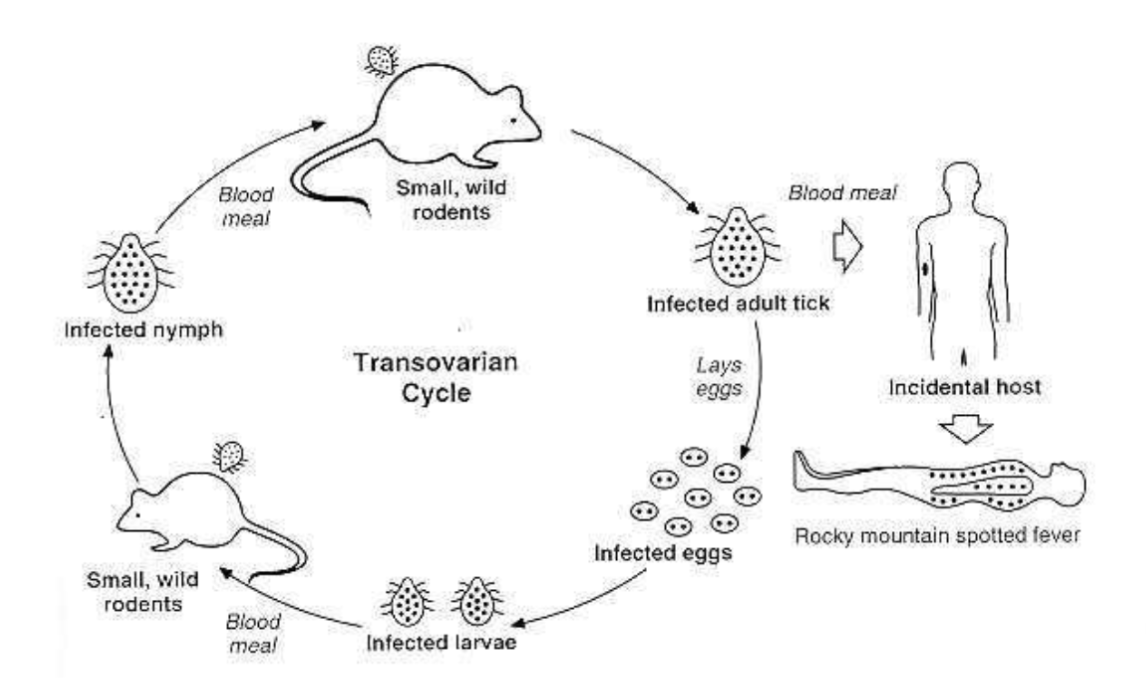
Rocky mountain spotted fever life cycle
Dermacentor sp.
Rocky Mountain wood tick (Dermacentor andersoni) in the western United States- Can also cause Colorado tick fever virus. American dog tick (Dermacentor variabilis) in the eastern United States. Also associated with the transmission of Francisella tularensis (tularemia) and causes tick paralysis in some cases.
Coxiella burnetii general characteristics:
Causative agent of Q (query) fever
Found in sheep, cattle and goats
Inhalation of or contact with the milk, urine, feces, or birth products of infected animals
High fever, severe headache, chills, diarrhea and pneumonia but no rash
Because its signs and symptoms mimic other infections, accurate testing is necessary for detection.
Rickettsia: typhus fevers: symptoms:
severe headache, chills, fever, rash caused by subcutaneous hemorrhage as Rickettsia invade the blood vessels.
Epidemic typhus general characteristics:
caused by R. prowazekii
transmitted by human lice
occurs in crowded areas causing epidemics
"camp fever" or "war fever."
high mortality rates in untreated cases
May develop into a latent infection with occasional relapses (Brill-Zinsser disease- It is a delayed relapse of epidemic typhus that occurs years after the initial ainfection. )
Brill-Zinsser disease:
A delayed relapse of epidemic typhus that occurs years after the initial infection
Endemic typhus (rat-flea typhus or murine typhus)
Caused by R. typhi
Transmitted to man by rat fleas
Similar but milder than epidemic thyphus
R. akari: Rickettsial Pox: general characteristics
transmitted by a mouse mite
papulovesicular rash, often preceded by an eschar (thick crust) at the site of the mite bite.
fever, malaise, and headache
rash
mild disease, usually not fatal.
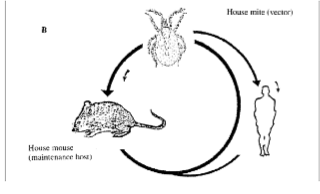
Orientia tsutsugamushi: scrub typhus:

Neorickettsia, Ehrlichia and Anaplasma species general characteristics:
Cause disease in wild and domesticated animals, especially dogs, and humans
Tick is the vector
Organisms parasitize macrophages or granulocytes
Cause ehrlichiosis
Disease characterized by leukopenia and thrombocytopenia
Organisms enter the WBCs and develop into a cluster known as a morulae
Morulae can resemble a berry or blackberry (see Fig 31-7)
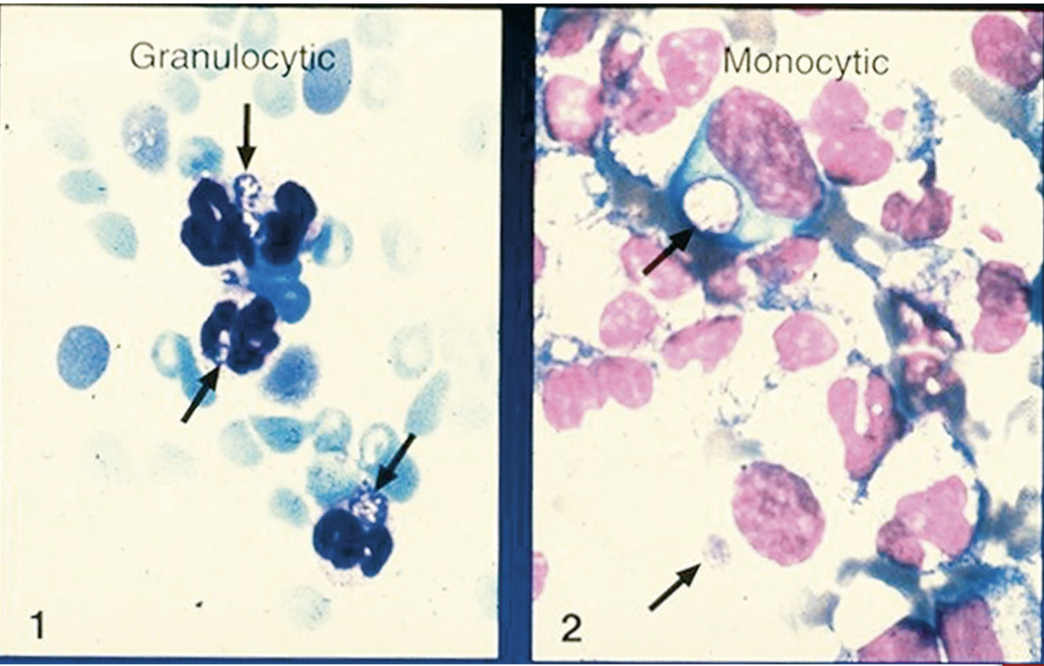
Human granulocytic anaplasmosis or ehrlichiosis:
Anaplasma phagocytophlium (human granulocytic anaplasmosis-HGA) and Ehrlichia ewingii (E. ewingii ehrlichiosis (HEE)) live in human granulocytes
HGA is more common in the Northeast and upper Midwest.
HGA- transmitted by Ixodes tick (scapularis and pacificus).
HEE transmitted by Lone start tick
Ehrlichia chafeensis general characteristics:
human monocytic ehrlichiosis-(HME)
Incidence highest in southeastern and south-central areas of US
Lives in human monocytes
Transmitted by lone star tick (Fig 31-8)
How to tell the difference between Ehrlichiosis and Anaplasmosis:
A rash is less common in anaplasmosis compared to ehrlichiosis.
Neorickettsia sennetsu general characteristics:
Mononucleosis-like illness in Japan and Malaysia
Infects monocytes and macrophages
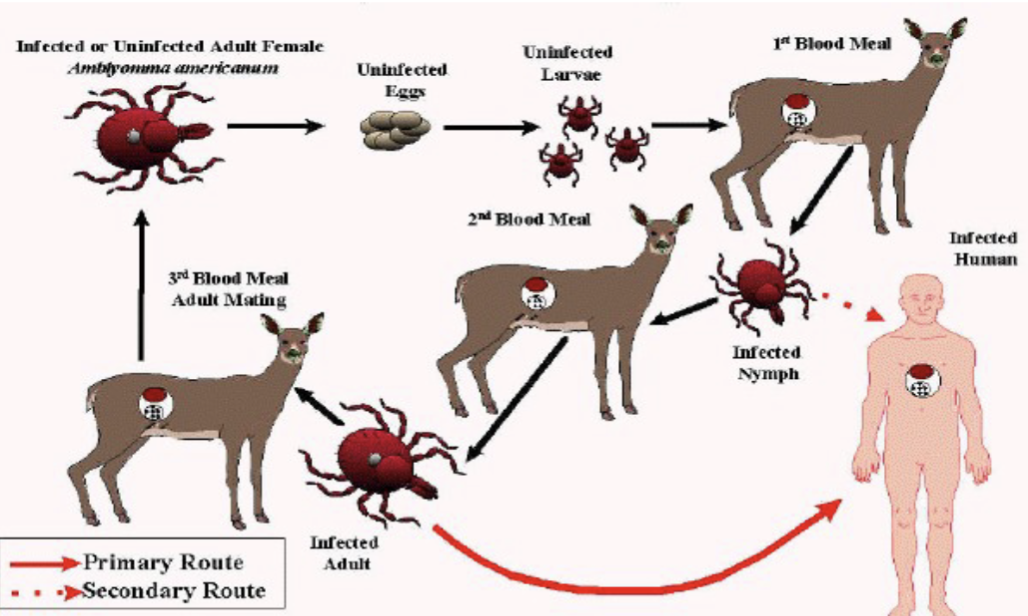
Rickettsia: Ehrlichia chaffeensis human monocytic ehrlichiosis (HME)
Rickettsia: Coxiella: B. burnetti:
causes Q fever (C.burnetti)
zoonotic disease (cattle, sheep, and goats are the primary reservoirs)
Organisms are excreted in milk, urine, and feces of infected animals; also in amniotic fluids and placenta
Highly infectious; resistant to heat, drying, and many common disinfectants (survive for long periods in the environment)
acquired by inhalation of infectious material
long incubation period; low mortality rate
causes atypical pneumonia with no rash (fever, chills, and headache)
Rickettsia diagnosis:
Serology
Indirect fluorescent antibodies-Indirect immunofluorescent assays demonstrating a four-fold rise in titer are most common.
Direct detection of Rickettsia in tissues
Giemsa stain
Wright stained blood smears examination for ehrlichiosis
Least sensitive method
May be attempted to examine for presence of morulae, cytoplasmic vacuoles in which Ehrlichia species grow, in peripheral WBCs.
Gimenez stain
Immunohistological stain
Direct fluorescent antibody test
DNA detection methods-Molecular Techniques/PCR
Effective but cost-prohibitive-Intraperitoneal inoculation of blood in laboratory mice.
Required for detection of N.sennetsu
General properties of viruses:
Smallest infectious agent known
Obligate intracellular parasites
Classified based on genomic nucleic acid
RNA or DNA but not both
Single stranded or Double stranded
Dependent on host cells for reproduction, as their cellular makeup lacks machinery for metabolism
Method by which they reproduce is known as viral replication and is unique to viruses
Virions:
virus particles which may consist of viral genome and its capsid only, or addition of the envelope
Capsid:
Outer protein shell of a virus
May be spherical (icosahedral) or helical
Nucleocapsid:
Protein capsid and nucleic acid
Envelope:
Bi-lipid layer surrounding the nucleocapsid of enveloped viruses
Virus structure: size and shape:
range 20-300nm in diameter. The shape of the virus particle is determined
by the arrangement of the repeating subunits that form the protein coat (capsid) of the virus.
Virus structure: viral nucleic acid:
Genome located internally and can be either single or double stranded DNA or RNA.
Viral capsid and Symmetry:
Nucleic acid is surrounded by protein coat called capsid made up of units called
capsomere. The structure with nucleic acid is called nucleocapsid.
Nucleocapsid had 2 forms of symmetry:
Icosahedral, capsomeres are arranged in 20 triangle called icosahedron. Icosahedral
nucleocapsid can be enveloped or naked.
Helical, capsomeres are arranged in hollow coil that appears rod shaped.
The advantage of identical subunits:
1) it reduces the need for genetic information
2) it promotes self- assembly, i.e., no enzyme is required
Advantages of viral proteins:
Capsid- protects the genetic material andmediate the attachment to the receptors on the host cell surface. This determines specificity.
Outer viral protein- induces antibody and activate cytotoxic T cells to virus infected cells.
Internal viral protein: Depending on the virus some have a DNA or RNA polymerase.
Tegument protein: located between nucleocapsid and the envelope. These control viral and cellular processes.
Super antigens: similar to super antigen produces by bacteria, such as toxic shock
Outer viral protein:
induces antibody and activate cytotoxic T cells to virus infected cells.
Internal viral protein:
Depending on the virus, some have a DNA or RNA polymerase
Tegument protein:
located between nucleocapsid and the envelope. These control viral and cellular processes.
Super antigens:
similar to super antigen produces by bacteria, such as toxic shock
Retro Viruses:
Enveloped viruses, icosahedral capsid and two identical strand of single stranded, linear, positive polarity RNA.
A special group of RNA viruses that carry a reverse transcriptase enzyme as part of their genetic makeup.
Capable of transcribing their RNA into DNA and then integrating that DNA into host cell genome.
Opposite to normal flow of genetic material which is for DNA to be transcribed to RNA and the RNA to be translated into proteins.
Host defense falls into 2 categories against viral infection:
Nonspecific
Specific
Nonspecific host defense:
Interferon and Natural Killer cells
Specific host defense:
Humoral and cell-mediated immunity. This is effective only later because it takes several days to induce an immune response
Viral Replication:
infect unicellular organisms such as protozoa, bacteria, and algae, as well as multicellular organisms such as plants and animals.
Viral nucleic acid or genome contains information necessary for directing infected host cells to synthesize viral progeny. Viral genome must be inserted into the host cell in order for replication to take place.
Steps of viral replication are discussed in detail in Chapter 31, and include attachment and absorption, penetration, uncoating, eclipse period, maturation, & release
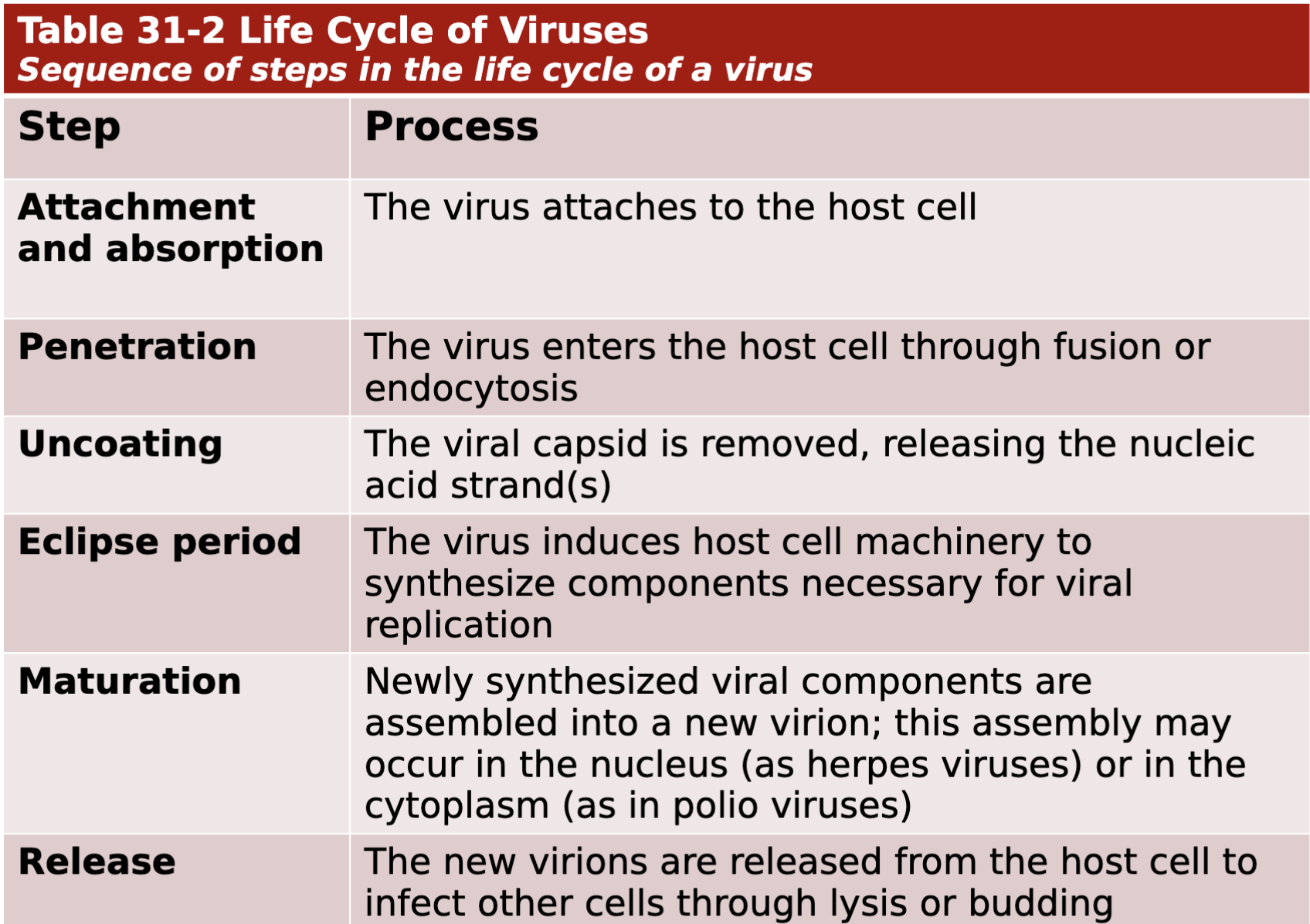
Sequence of steps in the life cycle of a virus
Lystic cycle:
Where the viruses replicate and rapidly take over the host cell, replicate, and cause the cell to burst
Lysogenic cycle:
Where the virus replicates and integrates their DNA into the host’s DNA and remain dormant until triggered to enter the lytic stage
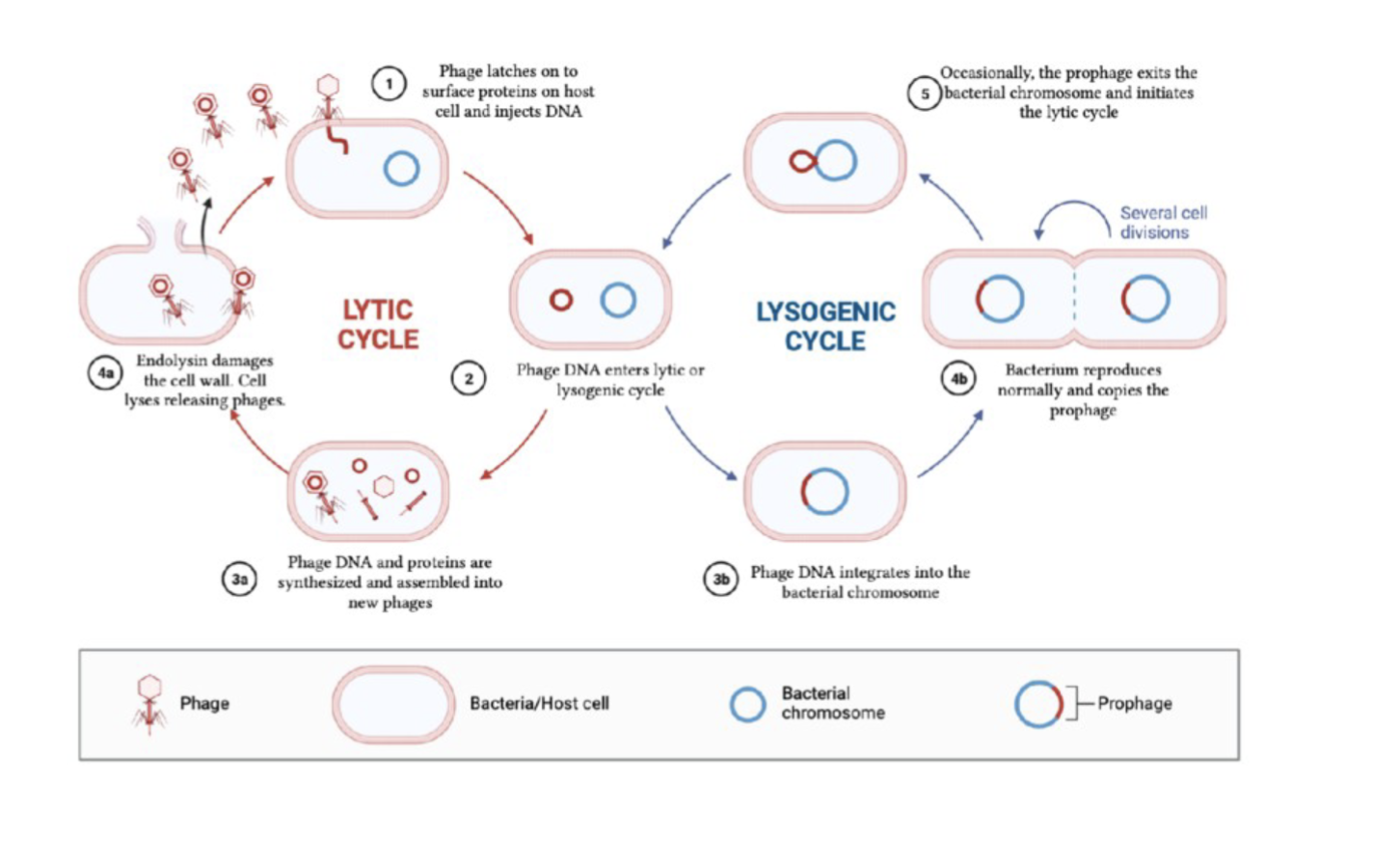
Lytic and Lysogenic Cycle
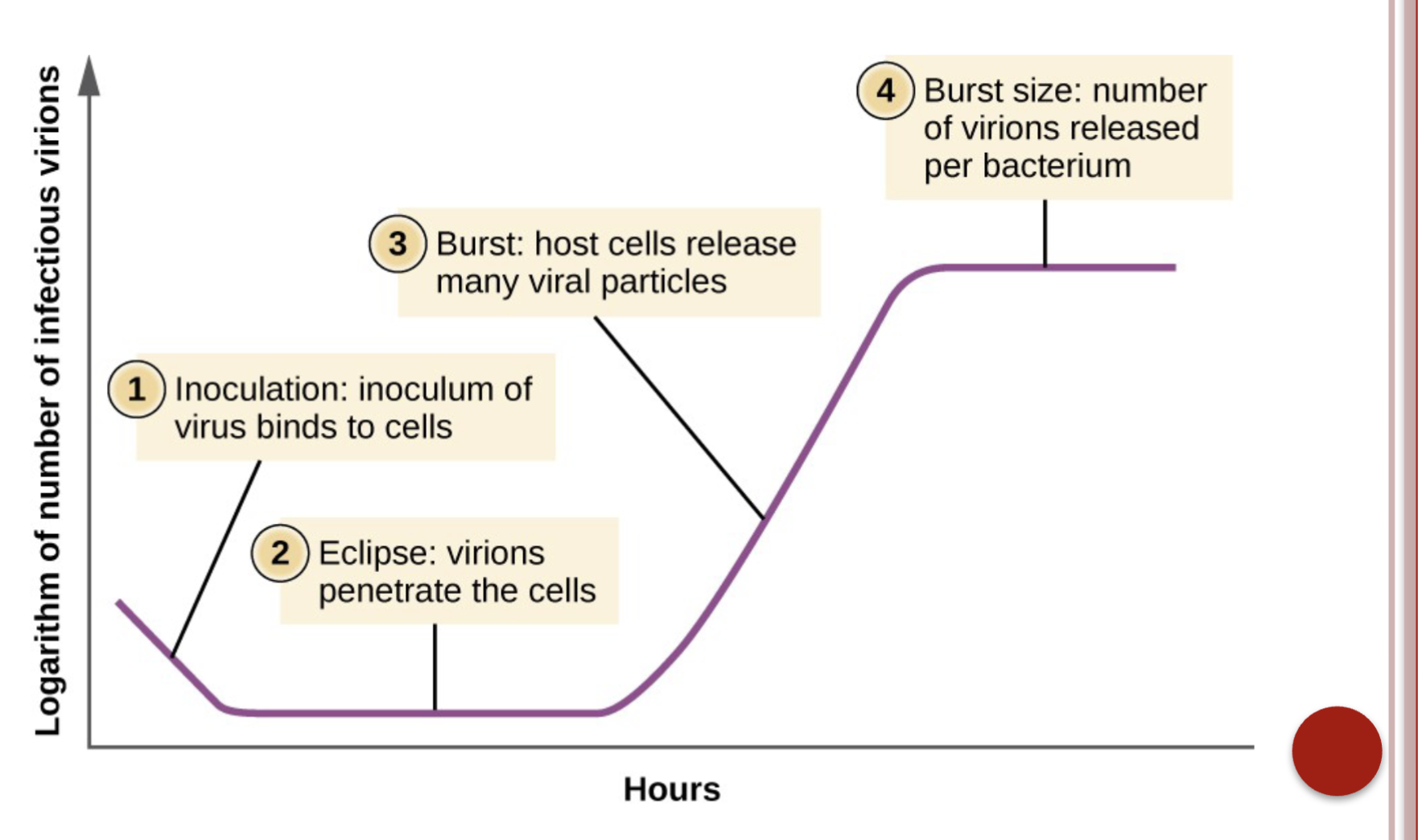
Phases of replication
Immunity to Viruses:
Production of interferons and complement
Activate natural killer (NK) cells to kill infected cells
IgA: prevents virus from binding to mucosal surfaces
IgG and IgM: able to coat and neutralize viruses outside the host cell
Antibodies also act as opsonins (proteins, like antibodies and complement proteins, that bind to pathogens or other targets, making them more easily recognized and consumed by phagocytes (immune cells) in a process called opsonization)
Cell mediated immunity recruits cytotoxic T lymphocytes to kill virus infected cells
Classification of Viruses: Taxonomy of Viruses:
Order (virales)
Family (viridae)
Subfamily (virinae)
Genus (virus)
Species
Classification of Viruses: DNA or RNA Viruses
Double stranded or single stranded
Circular or linear
Positive or negative sense
Classification of Viruses: Capsid structure:
Icosahedral
Helical
Diagnosis of Viral Diseases:
Cell methods
Embryonated eggs
Animal inoculation
Cell culture
Examine for cytopathic effect (CPE)
Necrosis, inclusion bodies, formation of syncytia, vacuolization
Cell lines
Primary can only be subcultured one or two
times
Low passage or diploid are limited to about 50
generations
Continuous can be propagated indefinitely- immortalized cells
Other ways to diagnose viral diseases (more common):
Direct detection of the virus
Immunostaining
Light microscopy
Electron microscopy
Molecular diagnostics
Gene amplification
Polymerase chain reaction (PCR)
Nucleic acid sequence-based amplification (NASBA)
Amplifies RNA instead of DNA
Uses reverse transcriptase to synthesize cDNA from RNA.
Branched DNA (bDNA)
Without amplification it detects nucleic acid DNA or RNA.
Good for viral load (e.g. HIV)
Quantifies mRNA
High throughput diagnostics- less prone to contamination.
Viral Serology:
Detection and measurement of antibodies
Radioimmunoassay
Enzyme immunoassay
Complement fixation
Latex agglutination
Immunofluorescent assays
Hemagglutination inhibition
Viral pathogenesis:
Viral replication may result in cytolysis and subsequent release of virus particles
Immune response destroys viral infected cells by:
Cytotoxic antibodies
Complement activation
Cell-mediated destruction
DNA Viruses: Classification System:
Devised by the ICTV (International Committe on Taxonomy of Viruses)
Seven families of DNA Viruses:
Herpesviridae
Poxviridae
Adenoviridae
Papovaviridae
Polyomaviridae
Parvoviridae
Hepadnaviridae
Herpesviridae general characteristics:
Linear dsDNA with a capsid and envelope
Herpes simplex viruses
Latent in the cells of the nervous system
HSV-1
Orofacial lesions called fever blisters or cold sores
HSV-2
Genital lesions
Both types have been isolated from both sites
Very common infection with up to 80% of individuals infected with HSV-1 and 20% with HSV-2
Herpes Simplex Virus clinical manifestations:
Virus hides in sensory neurons and reactivates with stress, foods rich in arginine, sunlight, upper respiratory tract infection
Tingling, burning or itching at site in advance of appearance of blister-like lesion
Neonatal infections via birth canal of mother with an active infection (TORCH panel).
HSV encephalitis rare but high mortality rate
Herpes Simplex Virus treatment:
Acyclovir, docosanol (Abreva), vavacyclovir, tromantadine, lysine supplementation
HSV diagnosis:
CPE develops rapidly in 1-3 days on A-549, RK, WI-38, ML and MRC-5 cell culture lines
Cells enlarge, round up and may lyse and disintegrate
Tzanck smear on direct specimens and examine for giant
multinucleated cells
Direct fluorescent antibody on clinical specimen or CPE
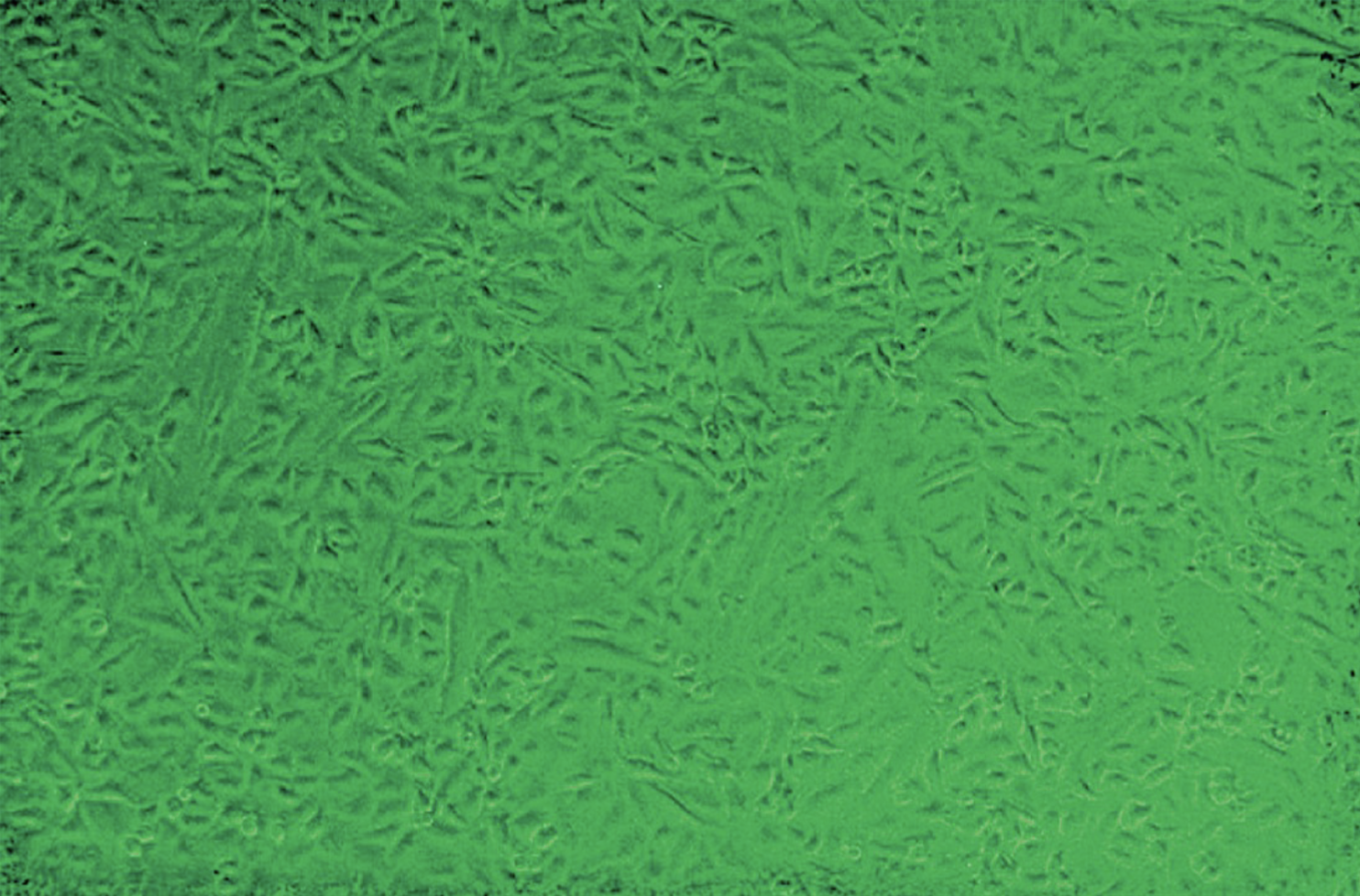
Unaffected vero cell line
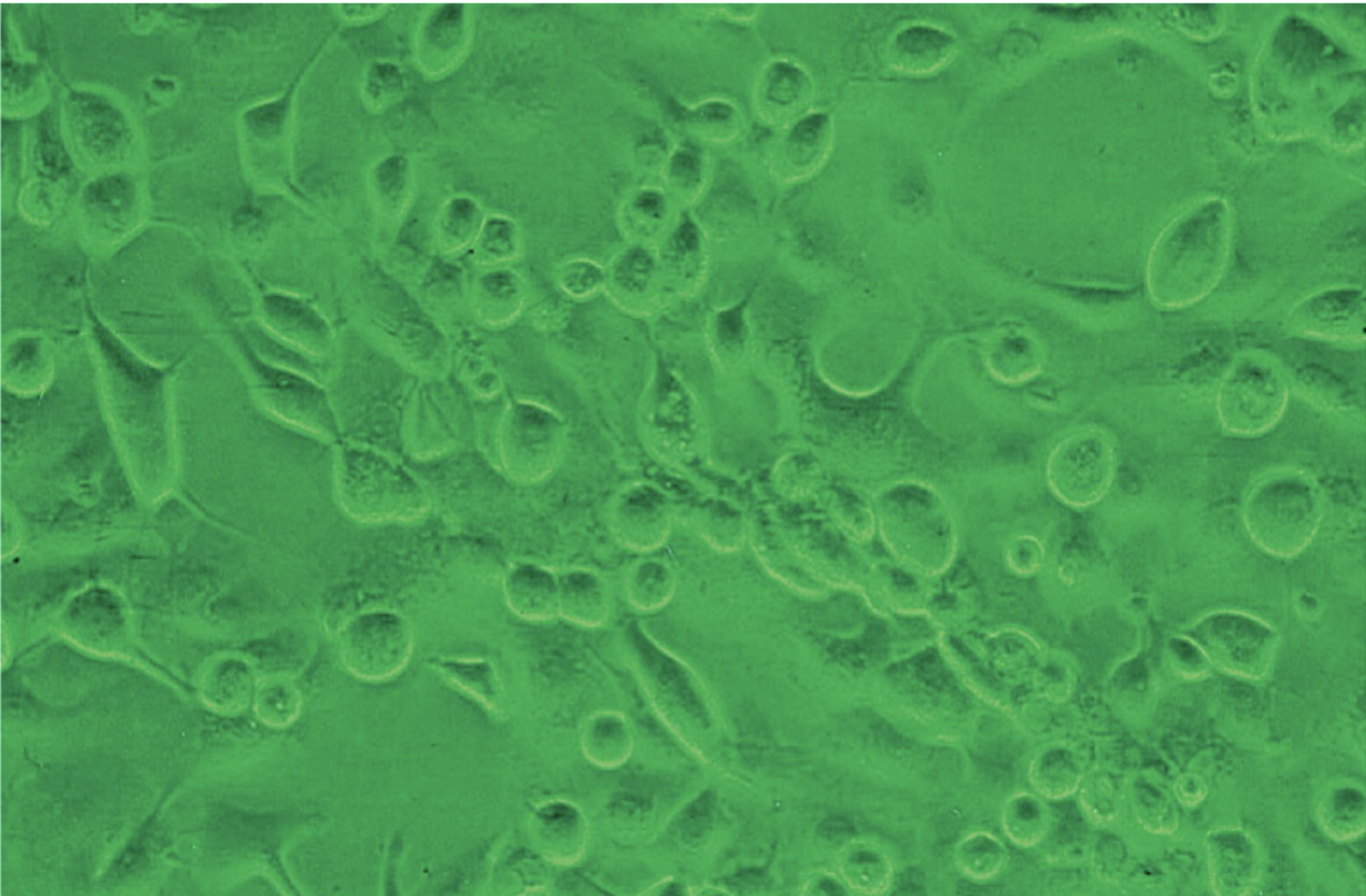
Vero cells that have enlarged and rounded up due to the presence of HSV-1
Torch panel:
group of tests used to screen newborns and, sometimes, pregnant women for certain infections that can cause birth defects in a baby if the mother contracts them during the
pregnancy.
The tests detect antibodies produced when exposed to the infectious diseases.
Toxoplasmosis
Other ( syphilis, parvovirus, zoster, HBV and HIV)
Rubella
Cytomegalovirus (CMV)
Herpes simplex virus (HSV)
Herpesviridae: Varicella zoster virus (VZU): general characteristics:
dsDNA with an envelope
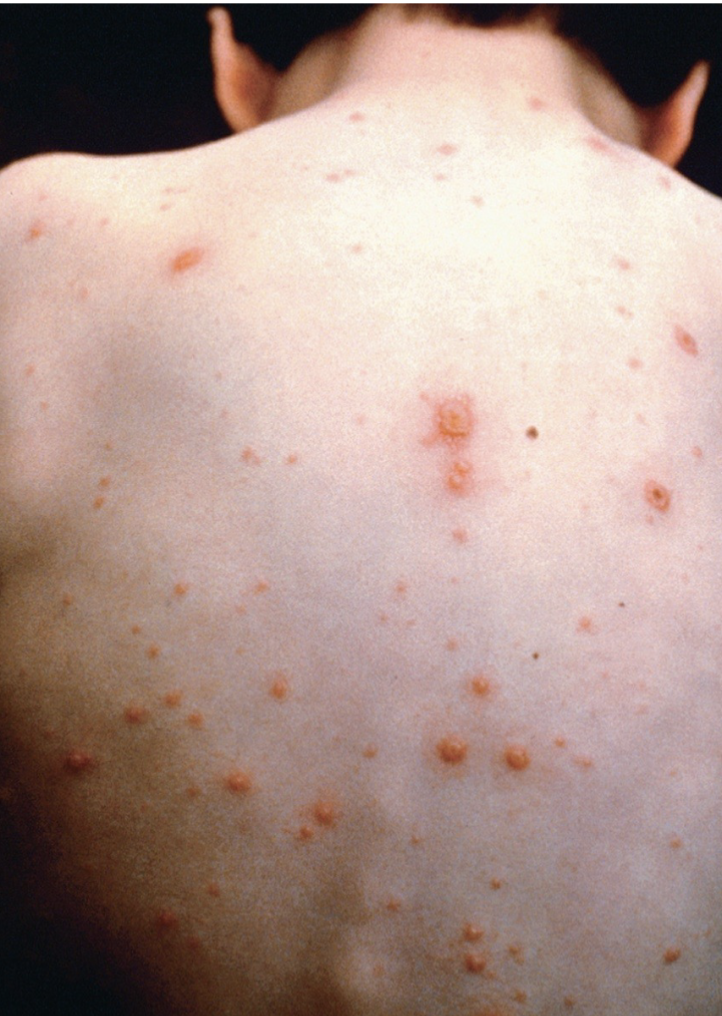
Causative agent of chickenpox
Characteristic vesicular rash and fever
Spread via aerosols
After infection, virus can remain dormant in nerves
Reactivation results in shingles (herpes zoster)
Spreads along the involved sensory nerve (Age- This is known as immunosenescence, and it increases the risk of shingles) and rash appears on one side of the body with severe pain
Increased risk of Post-Herpetic Neuralgia (PHN)
PNH Explained: PHN is chronic nerve pain that persists after the shingles rash resolves. It is caused by nerve damage from the VZV reactivation.
Age and Nerve Damage: Older adults are more likely to develop PHN because their nerves are more vulnerable to damage, and their bodies have a reduced capacity for repair.
Varicella zoster virus (VZV) treatment:
Anitviral drugs
Shingles vaccine
Varicella zoster virus (VZV): Diagnosis
Culture on human embryonic lung fibroblast cells such as MRC-5 and WI-38 as well as the lung cancer cell line A-549
Clusters of large, rounded cell within 1 week of culture
Tzank smear of lesion for giant multinucleated cells
Immunofluorescence on direct specimen and CPE
Shingrix Vaccine:
(recombinant zoster vaccine) is highly effective at preventing shingles and PHN, especially in older adults. It is recommended for adults over 50 or those with weakened immune systems.
Early treatment of Varicella zoster virus (VZV):
Starting antiviral therapy (e.g., acyclovir, valacyclovir) within 72 hours of shingles onset can reduce the severity and duration of the illness and may lower the risk of PHN. Pain Management for PHN
Herpresviridae: Epstein-Barr Virus (EBV): general characteristics:
Enveloped, dsDNA
Often oral transmission but also blood and perinatal
Targets oropharynx epithelial cells and CD21 receptor on B lymphocytes
Causes infectious mononucleosis- Kissing disease
Fever, sore throat, lymphadenopathy and hepatosplenomegaly
Associated with some lymphomas and cancers
Types of lymphoma associated with EBV:
Burkitt’s lymphoma
Hodgkin’s lymphoma
Diffuse large B-cell lymphoma (DLBCL)
Nasopharyngeal carcinoma
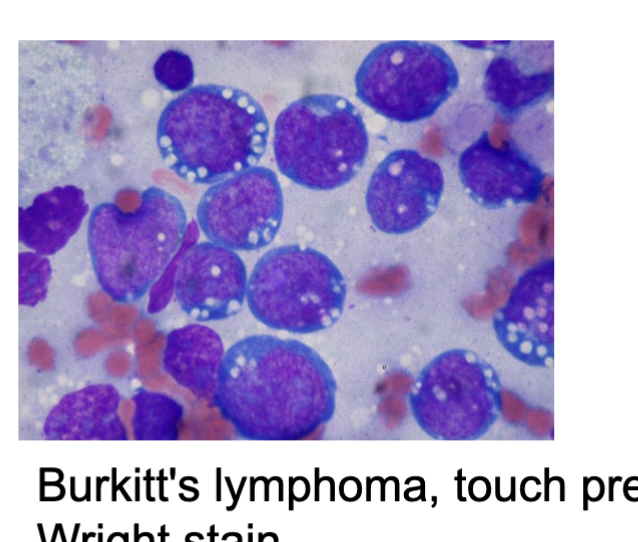
Burkitt’s lymphoma:
A rare but aggressive lymphoma that is particularly common in children and young adults in Africa and other tropical regions.
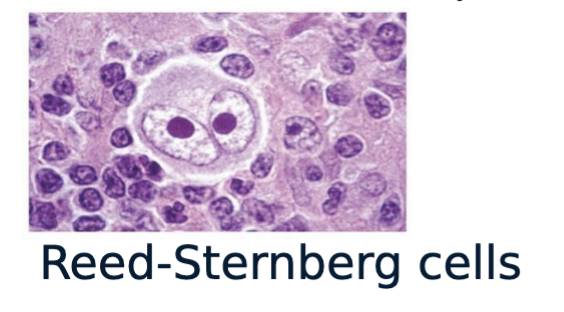
Hodgkin’s lymphoma:
A type of lymphoma that is characterized by the presence of Reed-Sternberg cells.
Diffuse large B-cell lymphoma (DLBCL):
A common type of lymphoma that can be EBV-positive or EBV-negative
Nasopharyngeal carcinoma:
A type of cancer that affects the nasopharynx (the back of the nose)
EBV lab diagnosis:
High WBC count, lymphocytosis (including atypical lymphocytes or Downey cells),
Positive monospot test- an increase in EBV-specific antibodies
Production of heterophile antibodies that cross react with antigens other than those that induced them
IgM antibodies that cross react with antigens other than those that induced them
React with sheep and horse red blood cells
Rapid monotest
Detection of EBV antibodies:
EIA, immunoblot, indirect immunofluorescence
Antigens such a early antigen diffuse (EA-D), early antigen
restricted (EA-R), viral capsid antigen (VCA) and Epstein-Barr nuclear antigen (EBNA) can induce antibody production
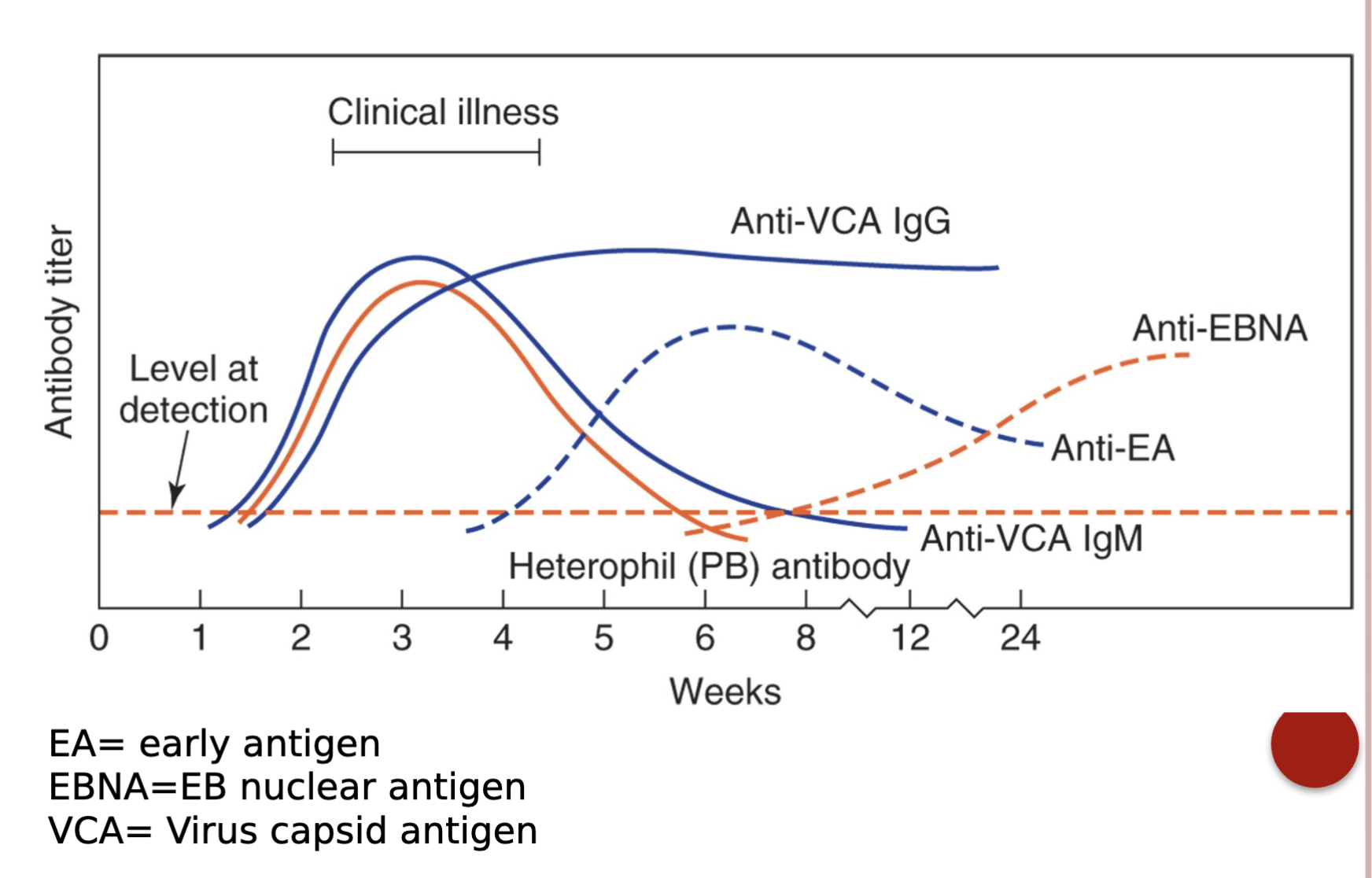
A serological profile of detectable antibodies to EBV infection
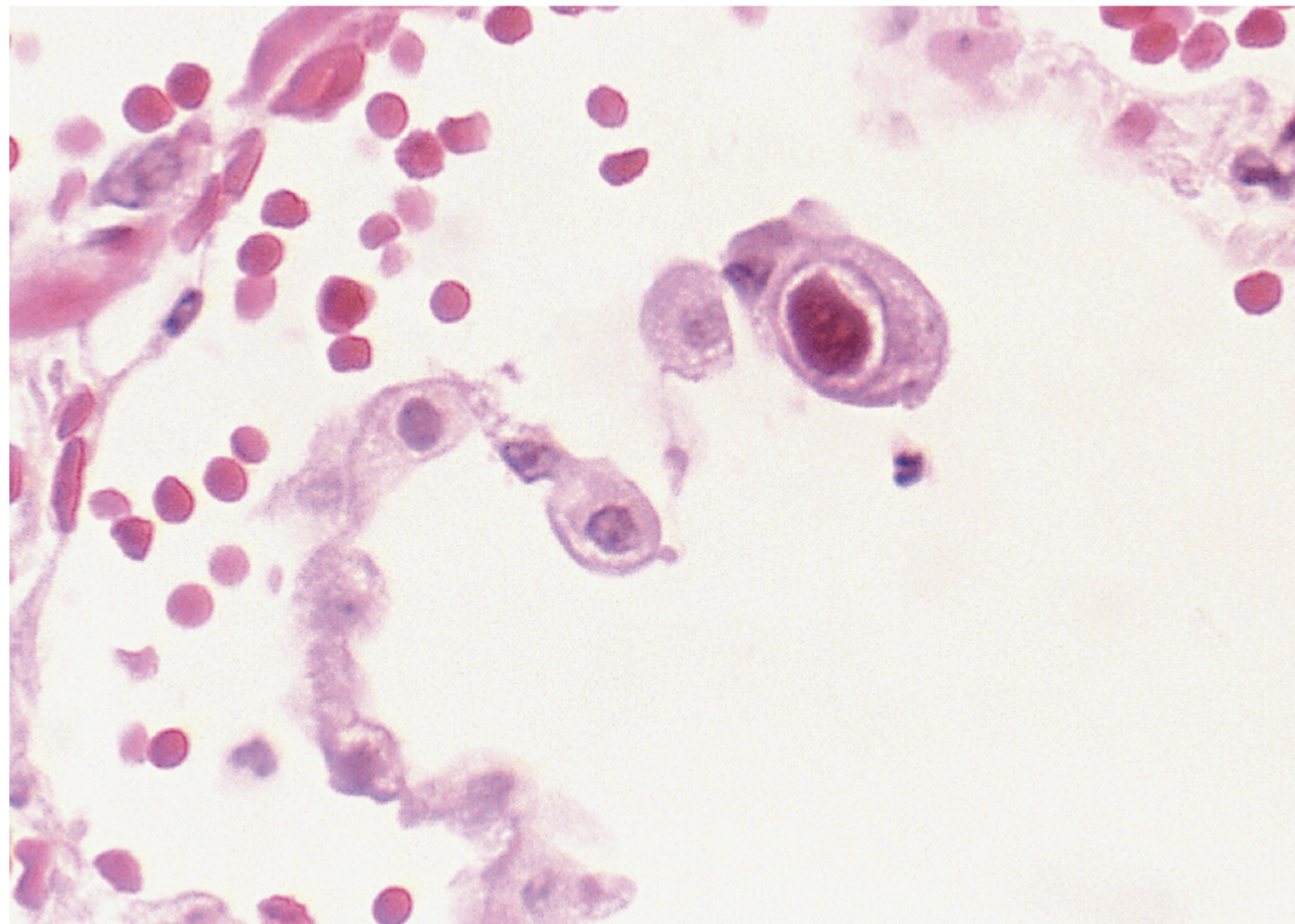
Herpesviridae: Cytomegalovirus (CMV): general characteristics
dsDNA
Transmission via breast milk, sexually, organ transplant, transfusion or contact with infected individual
Most common of congenital infections
Infection in fetus can be asymptomatic or fatal
Many individuals have latent infection and shed virus in
low numbers
Can be life-threatening in immunocompromised such as AIDS patients.
Newborn hearing screening- fail to pass should be tested
for CMV
CMV diagnosis:
PCR
Detection of viral nucleic acid in whole blood or plasma by EIA
Isolation of virus from human body tissue is confirmatory
In lung fibroblast cells, it produces large, basophilic, intranuclear inclusions called an “owl eye”
Serology tests can have cross reactions and cannot tell if active infection
Human herpesvirus 6 (HHV-6): general characteristics:
Very common pathogen found in saliva of over 90% of adults as a latent infection of the T cells
One of 2 etiologic agents of roseola infantum also known as exanthum subitum, sixth disease, or roseola
Rapid onset fever and rash when fever subsides
May be evidence to support its role in multiple sclerosis
Can reactivate with immunosuppression
Encephalitis, hepatitis, bone marrow suppression
Diagnosis is made clinically- no vaccine available and susceptible to antiviral drugs