(CHEMISTRY) GSCE COMBINED SCIENCE- TRILOGY (HIGHER TIER- 1H)
1/3
Earn XP
Description and Tags
Friday 17th May 2024, Mocks
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
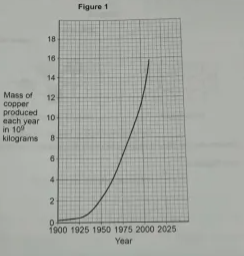
1.1) Copper is a useful material. Figure 1 shows the mass of copper produced between 1900-2010.
Give 2 conclusions that can be made from Figure 1. (2 marks)
1) Between 1900-1925, the mass of copper doesn’t increase much (slight amount)
2) After 1925, the mass increases rapidly. (faster rate)
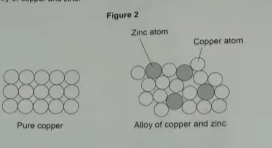
1.2) Mixtures of copper and zinc are heated to form alloys.
Figure 2 shows the structures of pure copper and of an alloy of copper and zinc.
Explain why the alloy of copper and zinc is harder than pure copper. (3 marks)
In pure copper, atoms are all the same size so the layers can slide over each other. In the alloy, the atoms are all different sizes so the layers are distorted and can’t slide over each other, making it harder.
1.3) A 5.25g sample of an alloy of copper and zinc contains 13.5% zinc by mass. Calculate the mass of copper in the 5.25g sample.
Answer to 3 significant figures. (4 marks)
13.5/100 × 5.25 = 0.70875 so 5.25- 0.70875 = 4.54
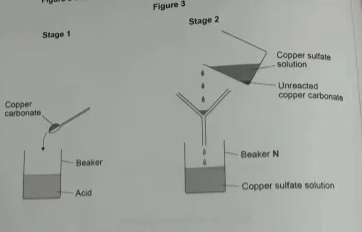
2) A student prepared copper sulfate by reacting an acid with excess copper carbonate. Figure 3 shows the first 2 stages in the preparation of copper sulfate.
2.1) What is the formula of the acid used to prepare copper sulfate? (1 mark)
H2S04