Chemistry - Physical Properties of Matter
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
properties (or characteristics) describe:
what can be observed by examining the substance
the way it behaves when brought into contact with other substances or exposed to sources of energy
extensive
those that depend on the amount of substance you have (eg. volume, weight, and mass)
intensive
those that do no depend on the side of the sample (eg. melting point)
Physical properties
characteristics that can be observed without the production of new substances
ex.: colour, odor, taste, density, melting and boiling points, electrical conductivity
physical properties of metals ex
malleable - capable of being changed, molded, shaped
ductile - stretched, hammered without breaking (wires)
luster - gloss, sheen
conductivity - ability to carry a current
physical change
involves the change of one or more physical properties=, but there is not change in the substances’s chemical composition or properties.
No new substances produced
eg.: Ice, water and steam have the same composition (88% O2, 11.2% H2)
ex.: changing physical appearance (crushing, blending), phase changes (solid, liquid, gas (boiling water, meting etc)), colour change, grinding a substance
chemical properties
characteristics that describe how the substance interacts (or fails to) with other substances to produce new substances
ex. iron rusting, failure of nitrogen gas to react chemically, flammability, toxicity, heat of combustion, chemical stability
chemical changes
any change that result int the production of one or more substances that differ in chemical properties and composition from the original substances
ex. souring of milk, burning of paper
changes in energy
includes absorption or energy and release of energy
every change, whether physical or chemical, involves an energy change
change in energy for physical changes tends to be less noticeable than those accompanying chemical changes
absorption of energy
a reaction that absorbs or used energy is said to be endothermic
(think endo → taking in the heat inwards)
physical - melting of ice, evaporation of water
chemical - formation of hydrogen gas and oxygen gas from water
release of energy
a reaction that releases energy is said to be exothermic
(think exo → heat being released → exo, outside)
physical - freezing of water, crystallization of a solid
chemical - burning wood
density
the mass of one unit volume of a substance
All substances have a specific, unique density
physical property of matter
density = mass/volume
grams per litre
can help us determine whether something will sink or float
compressibility
the ability of particles to move closer together
diffusion
movement of one substance through another
4 states of matter
solid
liquid
gas
plasma
solid
has a definite shape and volume
shape doesn’t depend on container
particles are tightly packed together, often in an ordered arrangement
will expand slightly when heated
usually incompressible
varying density ex. iron 7.87g/cm3, gold 19.3g/cm3, calcium 1.54g/cm3
liquid
particles of a liquid are in close contact, but arrangement is not rigid or orderly
flow freely, liquids take the shape of the container (some liquids pour easier than others)
volume remains constant, even if shape changes
almost/usually incompressible
will expand slightly when heated
can diffuse easily from areas of high concentrations to low concentrations
varying densities ex. mercury 13.53g/mL, water 1g/mL
gases
flows freely, will take the shape of the container
will expand to fit any volume
particles in a gas are much farther apart than solids and liquids
easily compressed
can diffuse easily from areas of high concentrations to low concentrations (ex. orange particles are easily diffused throughout the class room)
varying densities ex. hydrogen 0.089g/cm3, oxygen 1.43g/cm3, chlorine 3.21g/cm3
Plasma
a gaseous of mixture of positive ions and electrons
formed at higher temperatures greater than 100 million celsius when electrons are stripped from neutral atoms
very unstable
most common form of matter in the universe, comprising 99% of the visible universe, but are the least common on earth
DO NOT occur naturally on Earth except in the form of lightning bolts
ex. aurora borealis, lightening, florescent lights, and stars
neon sign - glass tubes filled with gas, electricity is turned on and charges the gas, creating plasma insides the tube and the colour depends on type of gas
kinetic
motion, comes from the greek word kinetos, ‘to move”
kinetic energy
"the energy an object has because of its motion
the main assumptions of the Kinetic Theory
i. the particles in a gas are considered to be small, hard spheres with an insignificant volume. (All particles are made of matter)
ii. the motion of the particles in gas is rapid, constant, and random. (Particles are in constant motion. The amount of motion depends greatly on the kinetic energy of a substance)
iii. all collisions between particles in a gas are perfectly elastic. (No change in kinetic energy takes place and particles bounce off each other without losing kinetic energy, only changing direction)
what is an ideal gas
a hypothetical gas composed of molecules that don’t attract nor repel each other
one that follows the gas laws at all conditions of pressure and temperature. Such a gas would have to conform precisely to the assumptions of kinetic theory
explain how “average kinetic energy” of a collection of particles is related to temperature
the particles in any collection of atoms or molecules at a given temperature have a wide range of kinetic energies
the average kinetic energy is used when discussing the kinetic energy of a collection of particles in a substance.
at any given temperature the particles of all substances have the same average kinetic energy
as the average kinetic energy increases, the temperature of a substance will rise
why is Kelvin temperature used when discussing the kinetic energy of a sample? What is the relationship between Kelvin and degrees Celsius?
the kelvin temperature is directly proportional to the average kinetic energy of the particles of a substance.
ex. helium at 200K has twice the average kinetic energy of helium at 100K
kelvin temperature
directly proportional to the average kinetic energy of the particles in a substance
K = C + 273.15
explain how the kinetic theory applies to liquids
liquids have kinetic energy, which means that they are always in motion
explain how the kinetic theory applies to solids
solids are made of matter, and their particles are in constant motion (they vibrate), which means that they also have kinetic energy
kinetic energy
energy due to motion of an object
potential energy
stored portion of energy
average kinetic energy
used when talking about the kinetic energy of a substance
temperature
a measure of the average kinetic energy of the particles of a substance
absolute zero
-273C or 0 Kelvin
there is a temperature at which all particles of a substance will stop moving
crystal structure
most solid substances are crystalline (clear, transparent, or sparkling like crystal, or made of crystals, or having the regular structure of a crystal)
in a crystal, particles are arranged into an orderly, repeating 3 dimensional pattern called a lattice
shape of a crystal reflects the pattern of the particles within the solid
there are 7 crystal systems that can be distinguished by the angle at which the faces of the crystal intersect
the 7 crystal systems that can be distinguished by the angle at which the faces of the crystal intersect
isometric
hexagonal
tetragonal
trigonal
orthorhombic
monoclinic
triclinic
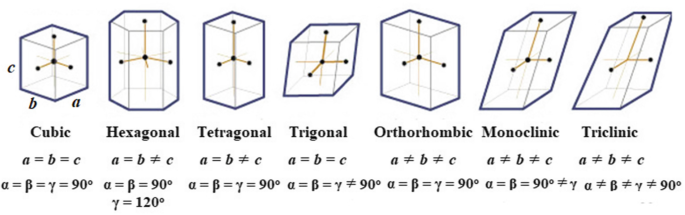
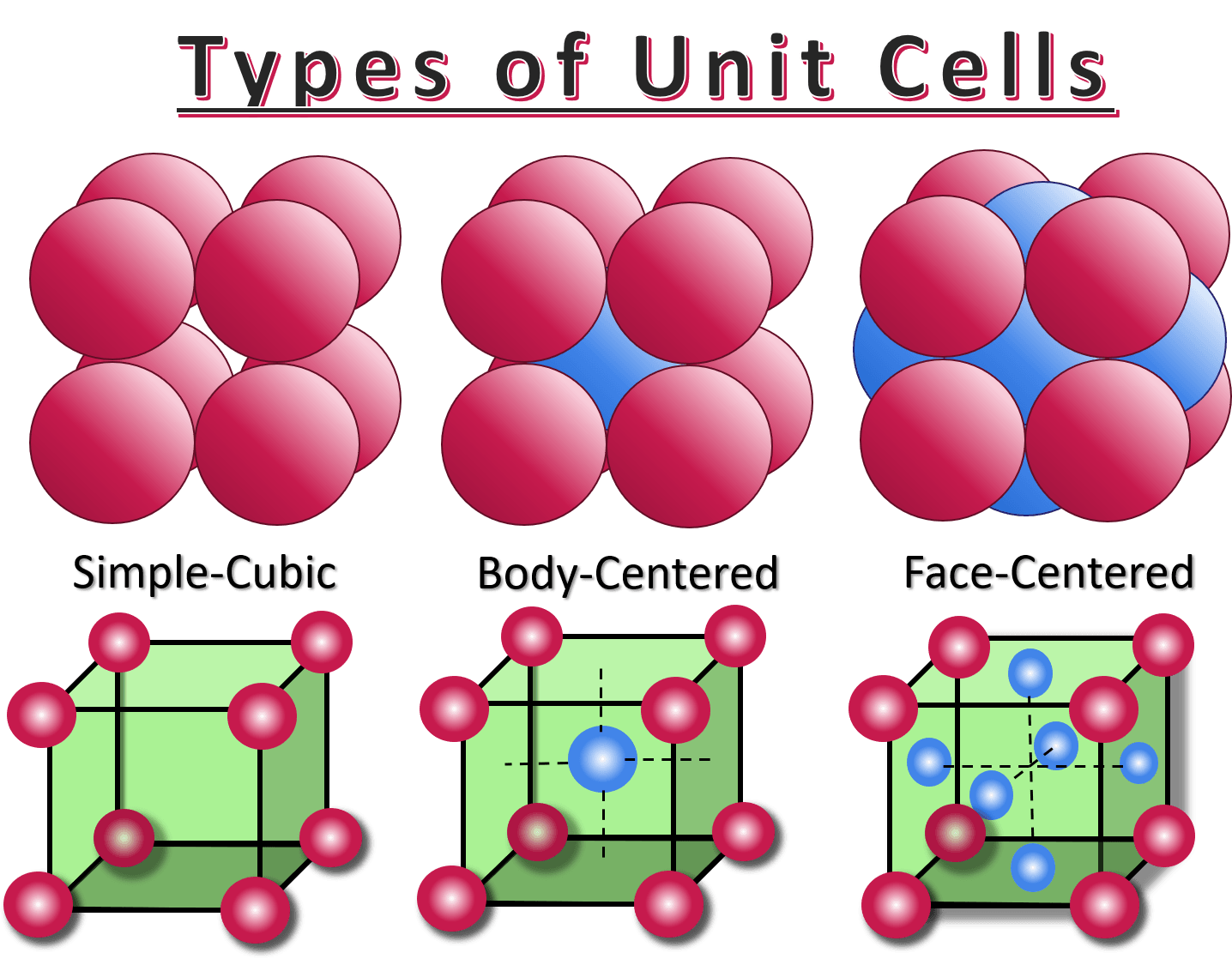
unit cell
the smallest group of particles within a crystal that retains the geometric shape of the crystal is known as the unit cell
the unit cell in a cubic crystal system may be simple cubic, face-centered cubic, or body-centered cubic
allotropes
2 or more molecular forms of the same element
ex. carbon
diamond - each carbon atom i bound to 4 others in tetrahedrons making it extremely rigid
graphite - sheets of linked hexagons, like chicken wire. stacked sheets have weak bonds holding them together making them slide easily and therefor soft and greasy
fullerenes - 60 carbon soccer ball shape, no known use yet. “buckmisterfullerene” 1996 Nobel Prize in Chemistry
allotropes of an element have different properties bc of their arrangement of the atom. Only a few elements have allotropes: carbon, phosphorous, sulfur, oxygen, boron, and antimony
amorphous solids
lacks an ordered arrangement of atoms (ex. glass)
atoms are randomly arranged
ex. rubber, plastic, peanut butter, and asphalt