Common IR/NMR Spectroscopy Values
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
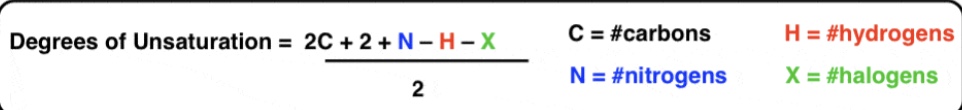
IHD / Degrees of Unsaturation
Aromatic C-C
1500-1600 cm-1
C=C
1550-1650 cm-1
C=O
~1700 cm-1; Very sharp peak
Alkyne (C triple C)
2100-2260 cm-1; Medium/weak intensity peak
Nitrile (C triple N)
2200-2260 cm-1; Sharp peak
sp3 C-H
2850-3000 cm-1
sp2 C-H
3000-3100 cm-1; Medium peak
sp C-H
~3300 cm-1
Alcohol (-OH)
3200-3500 cm-1; Broad, smooth peak
Amide (NH/NH2)
3300-3500 cm-1; Very sharp, teeth-like peaks; Number of peaks relates to substitution of amide. i.e. two peaks = NH2
1H NMR; C-H
0.5-1.5 ppm
1H NMR; Alkyne
2.0-3.0 ppm
1H NMR; R-OH
1.0-5.0 ppm; Position changes based on H-bonding
1H NMR; X-CH (X=F, Cl, Br, I)
3.0-5.0 ppm
1H NMR; Ether (R-O-CH)
3.2-4.0 ppm
1H NMR; Aromatic -H
6.5-8.5 ppm
1H NMR; Aldehyde (R-CHO)
9.0-10.0 ppm; Always a singlet (no neighboring H’s)
1H NMR; Carboxylic Acid (R-COOH)
10.0-13.0 ppm
13C NMR; Methyl (CH3)
10-20 ppm; sp2 and sp are slightly higher, respectively
13C NMR; C-X (X=halogen)
50-90 ppm
13C NMR; C-OR / C-OH
50-80 ppm
13C NMR; Alkyne (C triple C)
70-90 ppm
13C NMR; Alkene (C=C)
110-150 ppm
13C NMR; Aromatic C
110-160 ppm
13C NMR; Nitrile (C triple N)
110-130 ppm
13C NMR; Amide (R-CO-N-R2)
150-165 ppm
13C NMR; Ester (R-CO-OR)
155-180 ppm
13C NMR; Carboxylic Acid (R-COOH)
165-190 ppm
13C NMR; Aldehyde (R-CHO)
190-200 ppm
13C NMR; Ketone (R2-C=O)
200-220 ppm