Ap Bio: Unit 4- Cell Cycle
1/139
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
140 Terms
Cell division
fundamental process that allows organisms to reproduce, grow, and repair tissues. It involves the replication and distribution of a cell's genetic material (DNA) to produce two genetically identical daughter cells.
Genome
Organism’s entire genetic info
Chromatin
Complex of DNA and protein that serves as building blocks for chromasomes
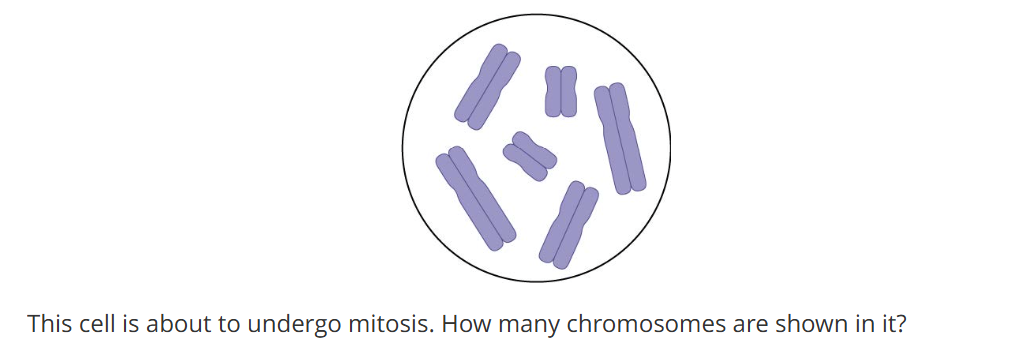
6
The mitotic phase
The phase of the cell cycle that includes mitosis and cytokinesis.
The cell cycle
the ordered sequence of events where a cell grows, duplicates its DNA and organelles, and then divides into two genetically identical "daughter cells," a process vital for growth, repair, and reproduction in organisms, primarily divided into Interphase (growth & DNA prep) and the M Phase (Mitosis/division)
Mitosis length compared to other stages
Short
interphase
90% of the cell cycle, seperated into G1, S, and G2
Phases of mitosis
Prophase, Prometaphase, Metaphase, Anaphase, Telephase
Prophase
The first stage of mitosis, in which the chromatin condenses into discrete chromosomes visible with a light microscope, the mitotic spindle begins to form, and the nucleolus disappears but the nucleus remains intact.
Prometaphase
The second stage of mitosis, in which the nuclear envelope fragments and the spindle microtubules attach to the kinetochores of the chromosomes.
Metaphase
The third stage of mitosis, in which the spindle is complete and the chromosomes, attached to microtubules at their kinetochores, are all aligned at the metaphase plate.
Anaphase
The fourth stage of mitosis, in which the chromatids of each chromosome have separated and the daughter chromosomes are moving to the poles of the cell.
Telophase
The fifth and final stage of mitosis, in which daughter nuclei are forming and cytokinesis has typically begun.
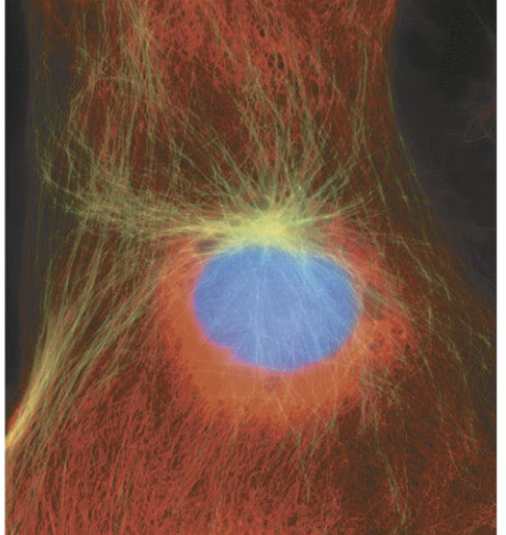
G2 of interphase
G2 of interphase

can you see chromosomes in interphase
no
How much chromatid for one chromosome
2
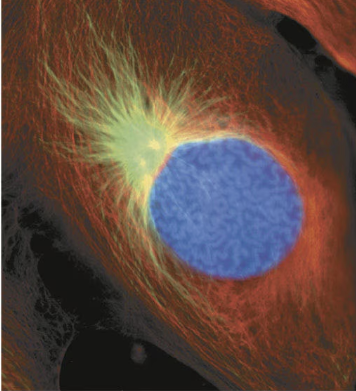
prophase
Prophase

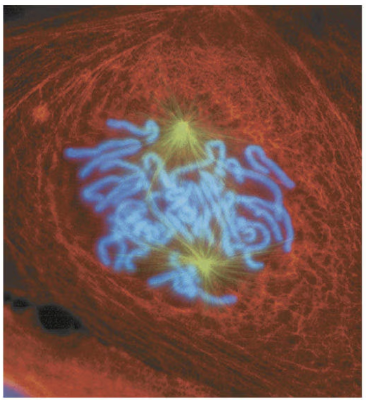
prometaphase
prometaphase
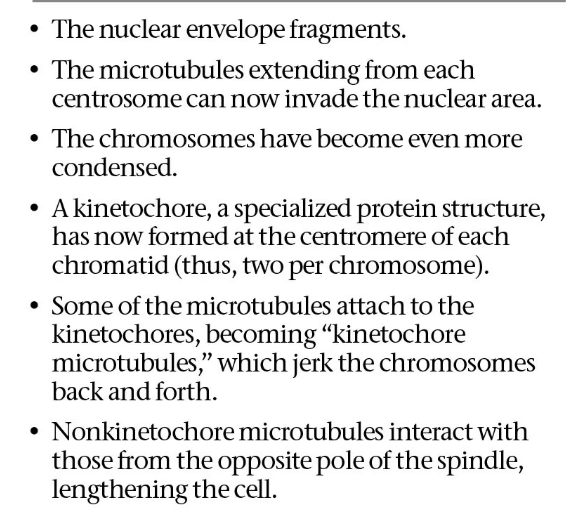
metaphase
metaphase

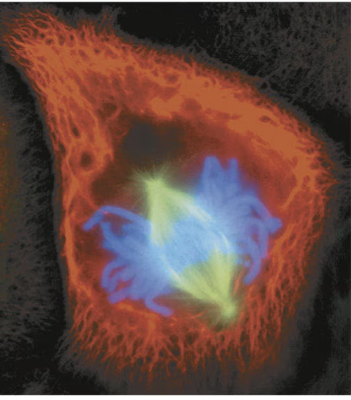
anaphase
anaphase

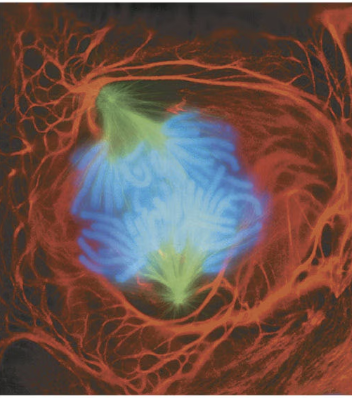
Telophase and Cytokinesis
Telophase

cytokinesis

Mitotic spindle
structure composed of microtubules which segregates chromosomes into the daughter cells during mitosis
Centrosome
primary microtubule-organizing center in animal cells. Regulates cell motility, adhesion and polarity in interphase, and facilitates the organization of the spindle poles during mitosis
microtubules
Maintain cell shape and provide rigidity, acting like internal tent poles. Forms the mitotic spindle, ensuring accurate separation of duplicated chromosomes into daughter cells.
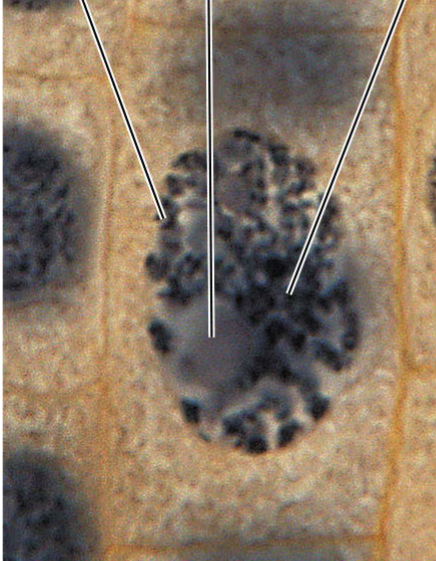
prophase
prometaphase

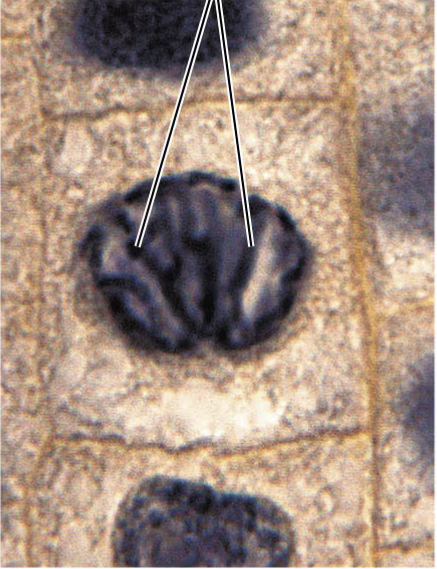
metaphase

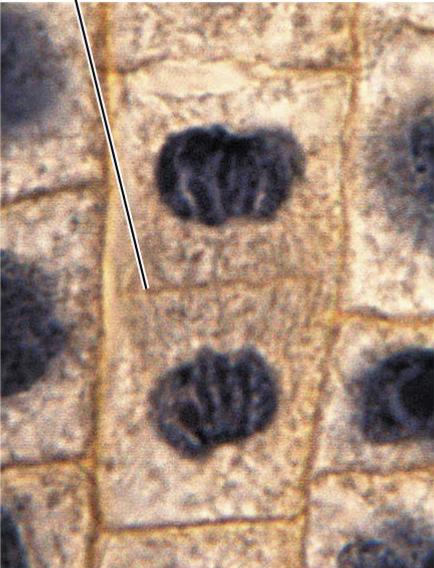
anaphase
telophase
Binary fission
A method of asexual reproduction in single-celled organisms in which the cell grows to roughly double its size and then divides into two cells. In prokaryotes, binary fission does not involve mitosis, but in single-celled eukaryotes that undergo binary fission, mitosis is part of the process.
The frequency of cell division varies in ____
type of cell
when do chromatids exist
Chromatids exist as a pair (sister chromatids) during the preparation and early stages of cell division (S phase, prophase, metaphase) and cease to be called chromatids once they separate and become individual chromosomes in anaphase
main phases of cell cycle
Interphase and Mitotic phase + (cytokinesis)
what all stages of interphase have in common
Cell grows through all the different phases of interphase
G1
Duplication of cell organelles, synthesis of proteins, RNA, and building blocks
Synthesis
Replication of genetic material and centrosomes
G2
synthesis proteins and RNA, makes organelles, reorganizes cellular contents
Interphase consists of how much of the cell cycle compared to the mitotic phase
a lot bigger
G0
Cell is outside the cell cycle, its just chilling
Prophase basically
cell prepare to divide. Chromatids condense, nuclear envelope no more
metaphase basically
Sister chromatids line up. microtubules attach to them
anaphase basically
microtubules pull apart chromatids
telophase basically
2 new nuclei
can a cell go through telophase without going through cytokinesis
yes, it would mean it has multiple nuclei
cytokinesis basically
cytoplasm splits
Animal cell cytokinesis
cleavage furrow is seen
Plant cell cytokinesis
vesicles are released to build cell plate to form new cell walls
G1 checkpoint
determines whether to complete the cell cycle. If no pass → go to G0
growth factor
adequate reserves (enough resources?)
check for DNA damage
G2 check point
Is DNA replicated, are there major errors? If problems with DNA are detected, cell cycle is halted to repair DNA
When is the G1 checkpoint?
end of G1, before S
When is the G2 check point?
end of interphase
CDK
Cyclin-Dependent Kinases, they phosphorylate target proteins- activating or deactivating them. Concentrations present in relatively stable amounts, but inactive until bound to a cyclin
M Checkpoint when?
during metaphase, triggers exit from mitosis/cytokinesis to begin G1
M Checkpoint
make sure sister chromatids are attached to the spindle microtubules
Cyclins
regulatory proteins that bind to CDKs, activating them and determining when they act. Concentrations fluctuate throughout cell cycle
what is a kinase
type of enzyme that adds phosphate groups to other molecules, like proteins, sugars, or lipids
What is MPF
Maturation-Promoting Factor)/M-phase Promoting Factor
Cyclin-CDK complex that triggers a cell’s pass the G2 checkpoint into the M phase. It is needed to activate proteins that are used in mitosis (ex. microtubules)
What checkpoints are there in the cell cycle
G1, G2, M
the 2 regulatory proteins that are involved in cell cycle control
cyclins and cyclin-dependent kinases (CDK)
How does MPF stop?
when there enough cyclin is degraded so MPF can’t form anymore
when do cyclins start accumulating
G1 of interphase
when do cyclins start degrading
Mitosis, around prometaphase
When does cytokinesis happen
starts around anaphase to end of telophase
Internal signals
Molecules and events occurring inside the cell that monitor the cycle's progress. (ex. cyclins, dna damage)
external signals
Signals from the outside environment that influence cell division. (ex. growth factors, cell crowding)
Density dependent inhibition
a crucial cellular process where normal cells stop dividing once they become crowded, triggered by physical contact with neighboring cells, preventing overgrowth and maintaining tissue structure
Anchorage dependence
the biological requirement for most normal cells to attach to a surface or extracellular matrix (ECM) to grow, divide, and survive, preventing uncontrolled growth and ensuring cells only proliferate in appropriate locations within a tissue
Cancer and the body’s control mechanisms
Cancers may not need….to grow and divide
growth factors
Why do cancer cells may not need growth factors to grow/divide
they may make their own growth factors
may convey a growth factor’s signal without the presence of a growth factor
may have a abnormal cell cycle control system
Cancer
uncontrolled cell growth resulting in a malignant tumor
transformation in cancer
when a normal cell becomes a cancerous cell
benign tumor
when abnormal cells remain at original site
malignant tumors
tumors that invade surrounding tissues, can metastasize
Hallmarks of cancer
sustains proliferative signaling
evade growth suppressors
active invasion and metastasis
enable replicative immortality
induce angiogenesis
resist cell death
oncogenes
mutated versions of normal genes (proto-oncogenes) that regulate cell growth, becoming permanently "on" and driving uncontrolled cell division, leading to cancer.
proto-oncogenes
normal genes essential for controlling cell growth, division, differentiation, and survival. They promote cell growth, regulate differentiation, and prevent apoptosis
telomerase genes
Reactivation of telomerase is a hallmark of most cancers, allowing them to grow uncontrollably. Provides instructions for the protein component of the telomerase enzyme, which adds DNA repeats (TTAGGG) to chromosome ends (telomeres) to prevent shortening and maintain cell division
p53 gene
a crucial tumor suppressor gene, often called the "guardian of the genome," that stops damaged cells from dividing and promotes DNA repair or programmed cell death (apoptosis) to prevent cancer
angiogenesis
the natural process of forming new blood vessels from existing ones, crucial for normal functions like wound healing, reproduction, and organ growth, but it also fuels disease, notably cancer, by supplying tumors with nutrients and oxygen, leading to rapid growth and metastasis
stability gene
the ability of an organism's or cell's genetic material (DNA) to remain consistent and unaltered across cell divisions, preventing mutations, rearrangements, and loss of genetic information
tumor suppressor gene
crucial genes that act as the "brakes" for cell growth, preventing uncontrolled cell division and tumor formation by repairing DNA, halting cell cycles, or triggering programmed cell death (apoptosis)
What would be the result of 2 mutated copies of a tumor suppressor gene in single-celled organism?
they reproduce uncontrollably
signal transduction pathway
series of steps that converts a signal on a cell’s surface into a specific cellular response
the most …. signal molecules bind to specific sites on ….. in the ….
water soluble, receptor proteins, plasma membrane
the 3 main types of membrane receptors
G Protein-coupled receptors
Receptor tyrosine kinases
Ion channel receptors
Paracrine signaling
cells signal to cells nearby, no direct connection, signal molecules need to diffuse
Endocrine signaling
cells signal to cells far away
the 3 stages of cell signaling
1) reception
2) transduction
3) response
reception
signal molecule to receptor
transduction
relay molecules in a signal transduction pathway after initial signal
response
cellular activity after transduction