Lecture 5 Inflammation, Hypersensitivities, and Tolerance
1/67
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
68 Terms
Three main goals of inflammation are:
1.Recruit immune defenses to the injured tissue
2.Limit the spread of infectious agents
3.Deliver oxygen, nutrients, and chemical factors essential for tissue recovery
Inflammation has 3 phases:
1. Vascular changes
2. Leukocyte recruitment
3. Resolution
1. Vascular changes
Injury or infection damages tissues then vasoactive molecules (like histamine, prostaglandins, leukotrienes, complement fragments) are released, these molecules cause vasodilation and increased vascular permeablity
2. Leukocyte recruitment
Cytokines attract white blood cells to the site (chemotaxis).
Neutrophils arrive first → they phagocytose (eat) pathogens.
Monocytes arrive later → mature into macrophages.
Both neutrophils and macrophages kill invaders and release more cytokines to recruit other leukocytes.
Margination and diapedesis
Margination
• Leukocytes slow as they roll along vessel wall
• Eventually leukocytes adhere to vessel wall
Diapedesis
• Leukocytes change shape
• Leukocytes squeeze out of vessel
Margination and diapedesis
Leukocytes undergo this to exit capillaries into surrounding tissue, occurs in leukocyte recruitment
3. Resolution
Blood vessels return to normal, Inflammatory signals decrease (Local tissue cells and leukocytes release cytokines and growth factors that shut down inflammation and promote healing)
Neutrophils and macrophages that are no longer needed undergo apoptosis (which forms pus)
Swelling decreases and healing increases
Chronic Inflammation
Response goes on too long past the injury or infection (not useful), it exacerbates tissue injury
Chronic Inflammation results in
• atherosclerosis,
• certain cancers, and
• progressive neurodegenerative disorders
Treatments for inflammation
Nonsteroidal anti-inflammatory drugs (NSAIDs) (e.g., aspirin, ibuprofen, naproxen)
Steroidal anti-inflammatory drugs(SAIDs) (e.g., cortisone, prednisolone)
Fever (pyrexia)
It is a systemic innate immune response
• Pyrogens—fever-inducing agents
• Trigger the release of cytokines
• Signal the hypothalamus to raise the body's baseline temperature
Low Grade Fever
Sometimes increasing your body temperature can help you fight off certain pathogens and also promotes tissue repair that.
Considered protective and can run its course
High Grade Fever
Dangerous, reaches 40.5 °C (105 °F)
Essential cellular enzymes and proteins will begin to denature and stop working
Hypersensitivities
are inappropriate immune responses (e.g., allergy and autoimmunity)
• Can be localized and therefore restricted to a given tissue
• Can be systemic and spread through the body and affect multiple tissues and organ systems
Hypersensitivities 4 types ACID
A Type 1 - Allergy, Anaphylaxis
C Type 2 - Cytotoxic
I Type 3 - Immune Complex
D Type 4 - Delayed Onset
-Each type can be triggered by drugs
Allergies type 1 Hypersensitivity
Include all allergies:
• Atopic asthma - allergy-based asthma
• Atopic dermatitis - inflamed and itchy skin condition also known as atopic eczema
Risk is genetic
Inhaled and ingested allergen
Sensitizing exposure
First exposure to allergen activates B cell which turn into plasma cells that produces IgE antibodies which have granules that contain histamine (doesn’t release histamine)
Post-sensitization exposure
allergen binds IgE → mast cell degranulation → allergy symptoms
Releases histamine and other chemicals
Induces degranulation
Degranulation
the process where immune cells (like mast cells, basophils, and eosinophils) release the contents of their granules (packets of chemicals) into the surrounding tissue
C Type 2 - Cytotoxic
Two Main Cytotoxic Mechanisms
It occurs when antibodies (IgG or IgM) bind directly to antigens on the surface of host cells.
This “tags” the cells as enemies → leading to their destruction.
Seen in conditions like hemolytic anemia, blood transfusion reactions, Rh incompatibility, some autoimmune diseases.
Two Main Cytotoxic Mechanisms
1. Complement-Dependent Cytolysis
2. Complement-Independent Cytolysis (Antibody-Dependent Cellular Cytotoxicity, ADCC)
1. Complement-Dependent Cytolysis
-Antibodies bound to a host cell activate the complement cascade.
-Complement proteins form the Membrane Attack Complex (MAC) → punches holes in the cell → cell lysis.
-Complement fragments also opsonize the cell → phagocytes eat it
2. Complement-Independent Cytolysis (Antibody-Dependent Cellular Cytotoxicity, ADCC)
-Antibodies bound to a host cell recruit leukocytes (like NK cells).
-NK cells bind to the antibody Fc region.
-NK cell releases perforin & granzymes, triggering apoptosis of the tagged cell.
Type II hypersensitivities are often characterized by cytotoxic reactions
-Goodpasture syndrome: Connective tissues of the kidney and lungs are attacked
-Autoimmune hemolytic anemia: Red blood cells are attacked when bound to drugs like cephalosporins and penicillins
-Rheumatic heart disease: Antibodies made against Streptoccocus pyogenes cross-react with the patient's heart valves
Type II - Blood Groups
Blood types referring to the presence of antigens on the surface of red blood cells• These antigens include:
• Carbohydrates: A, B, O
• Protein: Rh (rhesus factor) - indicated by a "+"
O blood type antigen
On every blood type, we all have O antigen
Difference between ABO and Rh
ABO are carbohydrates so they are everywhere in your body while Rh (D antigen) is a protein only in red blood cells, itll never be in your body unless you are Rh+
Rh-negative person only develops
this antibody after exposure to Rh-positive blood
• Usually from transfusion, or through placental exposure during a pregnancy
Incompatible transfused red blood cells cause
a hemolytic transfusion reaction
• Lyses red blood cells
• Could kill the patient
• Signs and symptoms occur within hours
• Fever, chills, lower back pain, chest pain, tachycardia, reducedblood pressuree
• No therapy to reverse a transfusion reaction or block it once it starts
• Supportive care to reduce kidney failure
O- blood type
the universal blood type
Rh factor incompatibility during pregnancy
may lead to hemolytic disease of the newborn (HDN)
-no effective way to prevent HDN if mother is already sensitized
To prevent HDN
prevent Rh negative women from ever being sensitized to the Rh factor
• Rh(D) immunoglobulin(RhoGAM) is given
Rh(D) immunoglobulin(RhoGAM)
This is an antibody against the Rh factor (also known as D antigen)
I Type 3 - Immune Complex
Antibodies and soluble antigens bind to form antigen antibody complexes deposit on the tissue which causes inflammation
Examples of Autoimmune Type III Hypersensitivities:
1. Systemic Lupus Erythematosus (SLE)
2.Rheumatoid Arthritis (RA)
3. Poststreptococcal Glomerulonephritis
Systemic Lupus Erythematosus (SLE)
Attacks DNA histones, ribosomes, ribonuclease, proteins the ones with the stars
Systemic (gastrointestinal, lung, kidney, and thyroid issues); often manifests with rash across cheeks and nose, fatigue, joint pain, fever, or hair loss
Rheumatoid Arthritis (RA)
Attacks Rheumatoid factor
Severe arthritis; mainly in wrists and hands; can cause bone erosion that deforms joints
Poststreptococcal Glomerulonephritis
Attacks antibodies against Streptococci cross-react with proteins in the kidney
May develop after untreated Streptococcus pyogenes infection; antibiotics make it rare in developed countries; usually resolves in weeks to months but may progress to renal failure.
D Type 4 - Delayed Onset
Both autoimmune and nonautoimmune
-autoimmune --> Multiple Sclerosis
-nonautoimmune --> Contact dermatitis (poison ivy) and latex reactions
Contact dermatitis
• Caused by drugs, nickel, chromate, poison ivy toxin (pentadecacatechol)
• T cells are sensitized
• Secondary exposure to the same antigen leads to inflammation and generates an extremely itchy (pruritic) red rash
Tolerance
The ability of the immune system to recognize “self” and not attack it (non-autoimmunity)
-Achieved through two main mechanisms: central tolerance and peripheral tolerance
- T cell (thymus and bone marrow) and B cell (lymph nodes and other lymph tissue)
Apoptosis
cell programmed death and is the mechanism for both central and peripheral
Central Tolerance (thymus) T cell
Happens during lymphocyte development (in the bone marrow for B cells, thymus for T cells).
-Immature lymphocytes are tested:
a. If they recognize self-antigens strongly, they are deleted (via apoptosis = negative selection).
b. Only cells that don’t strongly react to self survive
Apoptosis prevents
autoimmunity early on
Peripheral Tolerance T cell
Happens after lymphocytes mature and enter circulation/tissues.
Some self-reactive cells escape central tolerance → peripheral tolerance controls them:
-Anergy
-Regulatory T cells (Tregs) suppress self-reactive cells
- Apoptosis
Anergy
cells become inactive if they recognize antigen without proper co-stimulation
Central tolerance Summary T cell
(in thymus/bone marrow) deletes self-reactive cells by apoptosis during development
Peripheral Tolerance Summary T cell
controls escaped self-reactive cells in tissues using anergy, Tregs, or apoptosis
Regulatory T cells (Tregs)
A special subset of CD4⁺ T cells, supress directly by starving effector T cells
Their main job is to suppress or "turn down" immune responses so the system doesn't overreact or attack self.
Central tolerance B cells
Occurs in bone marrow
Immature B cells tested against self-antigens.
If B cell receptor (BCR) strongly recognizes self:
-Apoptosis (clonal deletion), OR
-Receptor editing
Receptor editing B cells
B cell rearranges its receptor genes to try making a non-self-reactive receptor
Peripheral Tolerance B cells
If self-reactive B cells escape:
-Anergy: they become unresponsive.
-Apoptosis: repeated stimulation by self-antigen without T cell help leads to cell death.
-Regulation by T cells: lack of proper T cell signals prevents them from becoming active.
Kinetics of an Immune Response
1.Establishment of infection
2.Inductive phase
3.Effector phase
4.Memory phase
Kinetics of an Immune Response Summary
The immune response begins with innate defense, gets overwhelmed, then adaptive immunity clears the infection, and finally memory ensures future protection.
1. Establishment of infection
Pathogen invades, colonizes and replicates & is detected by innate immunity
2. Inductive phase
Pathogen numbers exceed ability of innate response to control it
3. Effector phase
Effector cells of adaptive specific response start clearing the pathogen
T and B cells
4. Memory phase
Memory response ensures long-lasting protection (pathogen cleared)
MHC molecules
APCs engulf pathogen then break them into peptides where they bind the peptides to their class complexes (class 1 = T cells, class 2 = B cells), and then the peptide-complex bond will allow B and T cells to recognize
M1
pro inflammation
M2
Anti inflammation
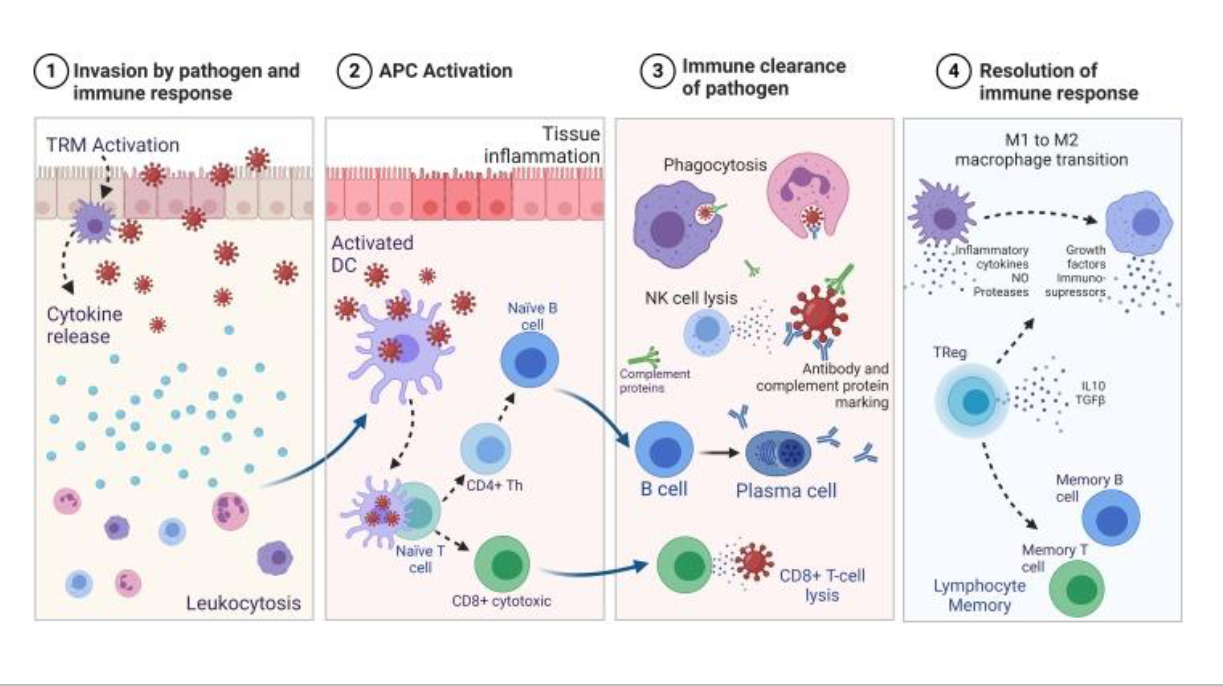
Different stages of infection cell invasion
1. invasion of pathogen and immune response
2. APC activation
3. immune clearance of pathogen
4. Resolution of immune response
1. invasion of pathogen and immune response
-Pathogen enters, colonizes, and begins replicating.
-Innate immunity responds first: barriers, complement, phagocytes, NK cells.
-Inflammation is triggered to slow pathogen spread.
2. APC activation
Antigen presenting cells will start bringing pieces of peptides (by engulfing) of the pathogens to MHC molecules which will then cause B and T cells to recognize to pathogen
3. immune clearance of pathogen
Adaptive immunity takes over:
-B cells → plasma cells → antibodies (neutralization, opsonization, complement activation).
-Cytotoxic T cells (CD8⁺) kill infected host cells.
-Helper T cells (CD4⁺) release cytokines to coordinate the immune attack.
Pathogen numbers decline.
4. Resolution of immune response
Once pathogen is cleared:
Macrophages will go from M1 to M2 anti-inflammatory, they are going to clean up and Tregs will push things to memory
Resolution summary
Every immune response must be followed by resolution, where inflammation stops, immune cells clear out, and tissues repair — otherwise chronic disease can develop.