Module 12: Glycolysis
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Glycolysis
occurs in the cytosol
extract energy oxidizing fuel molecules
Generate ATP
may generate reduced electron carriers and carbon skeletons that can be used in biosynthesis
converge on a few intermediates
multiple enzyme-catalyzed steps
regulated
primarily uses a few types of reactions
happens in all cell types
only source of metabolic energy for brain, kidney medulla, and rapidly contracting skeletal muscles, erythrocytes, sperm cells
How does glucose enter the cell?
Through glucose transporter protein (GLUT) transporter
How does glucose exit the cell?
As pyruvate either aerobically to the TCA Cycle or anaerobically as lactate
Stages of glycolysis: Reactions 1-5
Energy investment: high energy of ATP phosphoryl transfer used to generate low energy phosphoryl transfer compounds (only endergonic reactions!)
Stages of glycolysis: Reactions 6-10
Energy recovery: oxidation, coupled to phosphorylation using Phosphate and rearrangements convert these to high energy phosphoryl transfer compounds to make ATP (exergonic)
Step 1: Reactants to Products
Glucose and ATP —> Glucose-6-phosphate and ADP
- traps glucose because once it transforms, it is no longer glucose
Step 1: Enzyme
Uses hexokinase enzyme to phosphorylate glucose (activate glucose)
hexokinase is nonspecific and can phosphorylate several types of sugars
Mg2+ is a co-substrate (a type of coenzyme)
shields negative charges of phosphate group
large free energy change makes reaction irreversible
What is an inhibitor of hexokinase?
Glucose-6-phosphate
Step 1: Mechanism
A phosphate group is transferred from an ATP to a glucose (coupled reaction because of ATP hydrolysis)
Hydroxyl group on Carbon 6 performs a nucleophilic attack on phosphate in ATP to make it ADP
What kind of reaction is step 1?
Priming reaction through phosphorylation
Also a regulating step
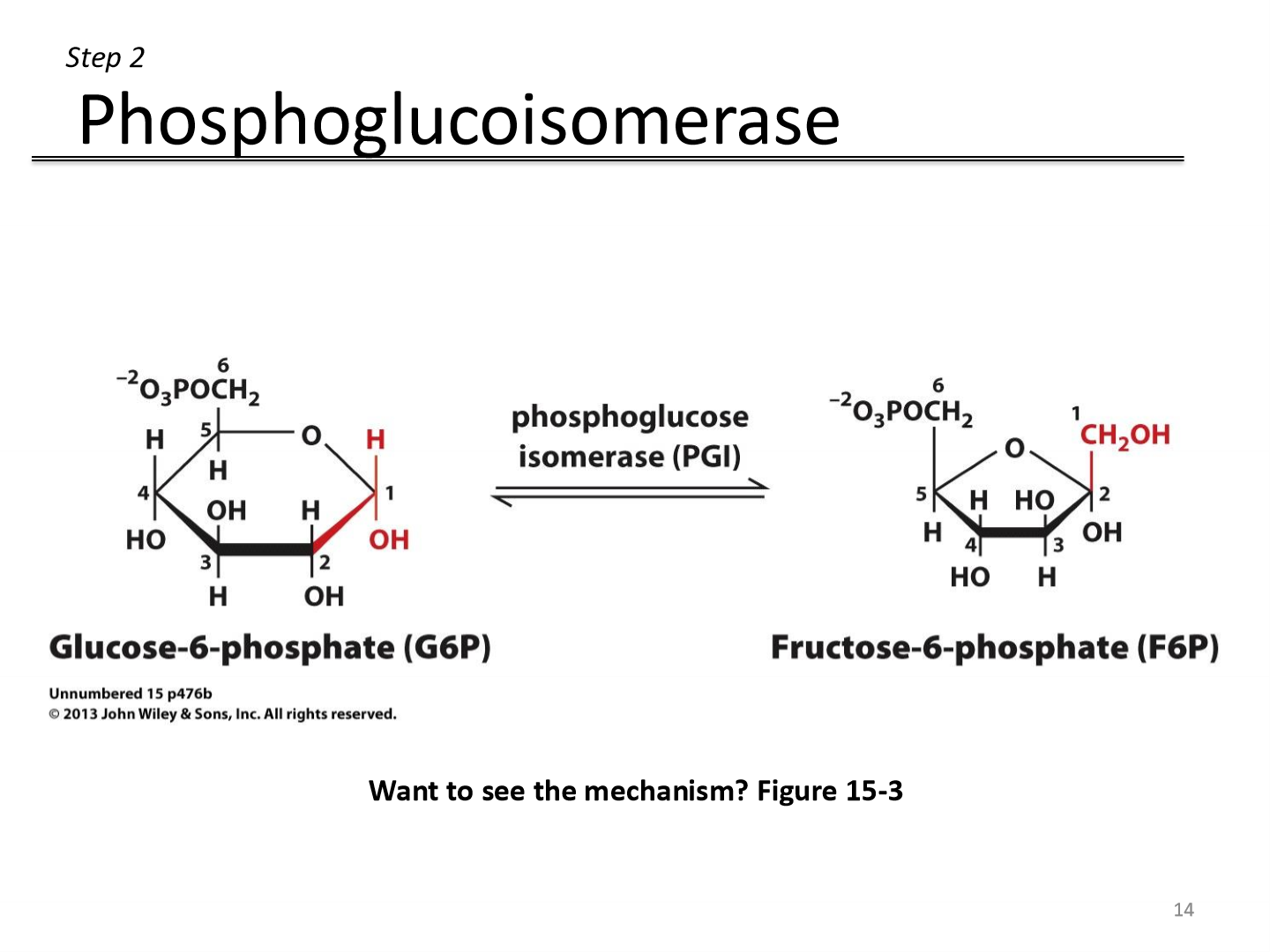
Step 2: Reactant to product
Glucose 6-phosphate ←→ fructose-6-phosphate
6-carbon sugar becomes a 5-carbon sugar
Step 2: Enzyme
Uses phosphoglucose isomerase enzyme
Step 2: Mechanism
- Involves general acid-base catalysis where an enzymatic acid, probably of a Lys residue, catalyzes ring opening
- Substrate binding
—> Acid-catalyzed ring opening: bond between O-C attacks an H+ atom on an acid, breaking the bond
—> base catalysis: base attacks the H+ on ‘2 carbon, double bond forms between carbon 1 and 2 from O-H bond
—> acid catalysis: double bond attacks another H+ from acid
—> base catalyzed ring closure: O- attacks ‘2 carbon to close the ring
—> product release
Step 3: Reactant to Product
Fructose 6-phosphate and ATP —> Fructose 1-6-bisphosphate
A phosphate group is transferred from an ATP to a fructose 6-phosphate
Step 3: Enzyme
Uses PFK-1 (phosphofructokinase-1) enzyme
when ATP is high, PFK is inhibited
when ADP and AMP levels are high, PFK is stimulated
Step 3: Mechanism
A phosphate group is transferred from an ATP to a Fructose-6-phosphate (coupled reaction because of ATP hydrolysis)
Hydroxyl group on Carbon 6 performs a nucleophilic attack on phosphate in ATP to make it ADP
What makes step 3 important?
The committed step, 1-6 bisphosphate can only go through glycolysis
fructose 1-6-bisphosphate is an activator for Pyruvate kinase (step 10)
rate-limiting step in glycolysis
highly regulated step
several inhibitors and activators
Step 4: Reactant to product
Fructose-1,6-bisphosphate —> Dihydroxyacetone Phosphate and Glyceraldehyde-3-phosphate
6-carbon sugar is split into two 3-carbon sugars
Catalyzed by aldolase to cleave FBP to form two trioses
Step 4: Enzyme
catalyzed by aldolase
Step 4: Mechanism
substrate binding through Lys (nucleophilic attack)
—> protonated Schiff base formation through Asp (covalent catalysis)
—> aldol cleavage (base catalysis)
—> tautomerization and protonation (acid catalysis)
—> Schiff base hydrolysis where substrate is released
Step 5: Reactants to products
The trioses formed from the last step (GAP and DHAP) are interconverted by an enediol intermediate and become ketoses, both Glyceraldehyde-3-phosphate
Step 5: Enzyme
triose phosphate isomerase
Step 6: Reactants to products
2 Glyceraldehyde 3-Phosphate + Pi <--> 1,3-biphosphoglycerate.
NAD+ <--> NADH
-The only redox reaction, carbonyl is being oxidized
-the first high energy intermediate
Step 6: Enzyme
Uses G3P dehydrogenase enzyme
NAD+ is a cofactor
Step 6: Mechanism
Substrate binding (nucleophilic attack)
—> active site thiol addition (covalent catalysis)
—> dehydrogenation (oxidation)
—> phosphate binding (nucleophilic attack)
—> product release and NADH/NAD+ exchange
What kind of reaction is step 6?
Oxidative
What is an inhibitor for step 6?
NADH
Step 7: Reactants to Products
1,3-bisphosphoglycerate + ADP —> 3-phosphoglycerate + ATP
Step 7: enzyme
Uses phosphoglycerate kinase enzyme
What is step 7 classified as?
Pay-off step because we gain 2 ATP
Step 8 Reactant to product
3-phosphoglycerate ←→ 2-phosphoglycerate
Step 8: Enzyme
phosphoglycerate mutase
Mutase: an enzyme that catalyzes the transfer of a functional group from one position to another
Step 9: Reactant to Product
2-phosphoglycerate —> 2 phosphoenolpyruvates
Step 9: Enzyme
enolase
Step 9: Product
2 phosphoenolpyruvate (PEP), a phosphorylated molecule with higher transfer potential than ATP
Step 10: Reactants to products
2 PEP + 2 ADP --> 2 Pyruvate + 2 ATP
Step 10: Enzyme
Uses pyruvate kinase enzyme
Step 10 Inhibitors and Activators
ATP is an inhibitor
activation by FBP and PEP
Net production of Glycolysis
2 ATP
2 NADH
2 Pyruvate
Why does glycolysis occur in steps?
Not all energy is dissipated as heat, rather it is stored in carrier molecules ATP and NADH. Energy can then be extracted in usable amounts
Fate of Pyruvate with oxygen (aerobic)
it becomes Acetyl CoA
it goes through the TCA cycle in the mitochondria
it becomes CO2 and H2O
Fate of pyruvate without oxygen: Alcoholic Fermentation
process to become ethanol and CO2
enzymes: pyruvate decarboxylase and alcohol dehydrogenase
Homolactic fermentation
it is converted to lactate by its gain of electrons form NADH (reduced to NAD+)
2 Pyruvate + 2 NADH —> 2 lactate + 2NAD+
lactate dehydrogenase
redox reaction
used to regenerate NAD+
Low NAD+ stops glycolysis
Regulation steps in Glycolysis
Very negative delta G
1st step —> hexokinase
3rd step —> Phosphofructokinase
10th step —> pyruvate kinase
PFK
Substrates = fructose-6-phosphate and ATP can only bind to substrate site
Allosteric activators = AMP, ADP, Fructose-2-6-biphosphate
can only bind to regulatory site
Allosteric inhibitors = ATP, Citrate, PEP
can only bind to regulatory site
PFK Allostery
ATP stabilizes T state
F6P and ADP stabilizes R state
Glutamate flips to the R state and becomes positive
This attracts F6P because it is negative
Substrate Cycle
Fructose 1-6-bisphosphate —> Fructose-6-phosphate
Fructose 1-6-BisPhosphatase is the enzyme
phosphatases catalyze the removal of a phosphate group
Gluconeogenesis
formation of glucose from noncarbohydrate sources in the liver
- maintains low glucose levels
Obesity
cause by the failure to maintain the input and output of energy, mainly from fructose, also from substrate cycling
Fructose
- can be converted to glycogen as well
- liver absorbs almost all the fructose in our diet so our muscles rarely receive it
- appears to have a greater tendency to be metabolized into triglycerides for energy storage relative to glucose
Fructose skips the committed step and goes straight to the 5th step as glyeraldehyde-3-phosphate through glucokinase