Chem unit 2 vocab
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
Kinetic molecular theory
-Particles of matter are always in motion
relative kinetic energy of particles in solid
very low
relative kinetic energy of particles in liquid
low
relative kinetic energy of particles in gas
high
Relative particle spacing in solid
small
relative particle spacing in liquid
medium
relative particle spacing in gas
large
shapes of particles in solid
Constant/doesn’t change
shape of particles in liquid
varies/takes on shape of container
shape of particles in gas
expands or contracts/takes up entire space of container
Physical properties
characteristics of matter that can be observed or measured without changing the identity of the matter
melting
solid → liquid
vaporization
liquid → gas (heats)
deposition
gas → solid
freezing
liquid → solid
condensation
gas → liquid (cools)
sublimation
solid → gas
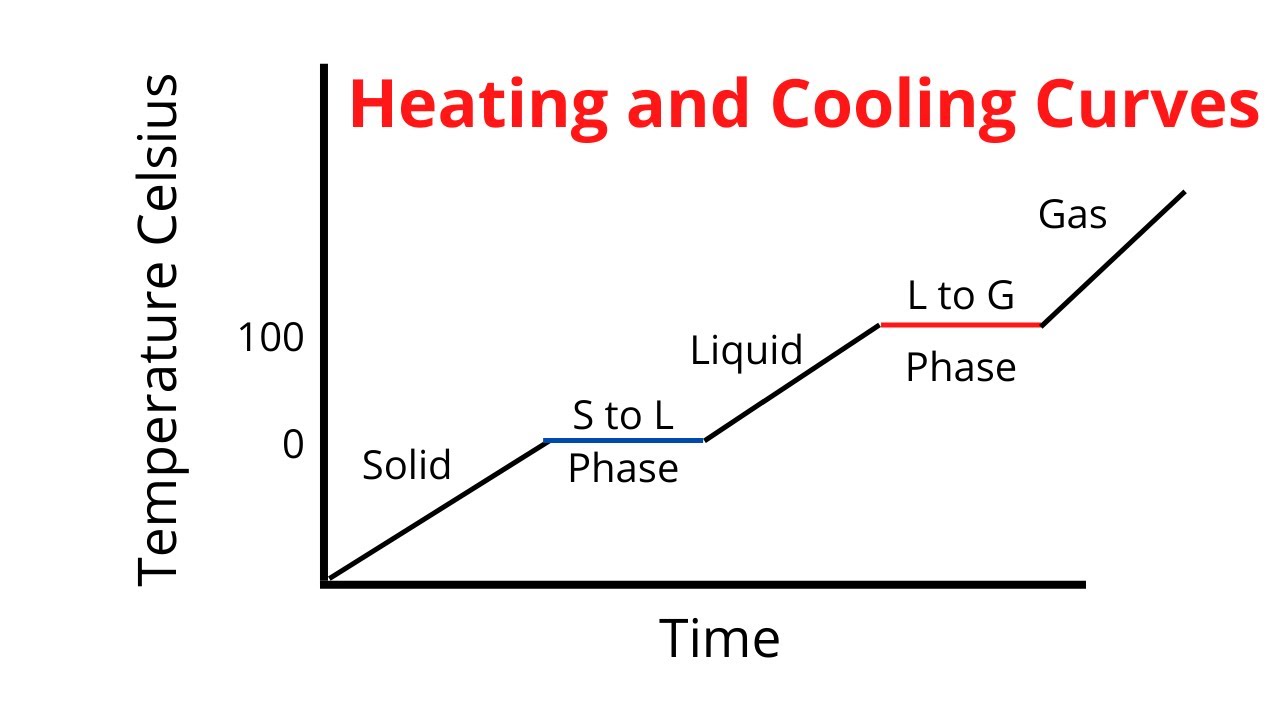
Phase change heating curve
Energy during phase change
→ kinetic energy remains constant
→ potential energy changes
chemical properties
are related to matter’s ability to undergo changes that transform one matter with a set of properties into another kind of matter with a different set of properties
→ chemical changes result in one or more kind of matter changing into a different kind of matter
law of definite composition
a given compound always contains the same, fixed ratio of elements and can be represented by a chemical formula
→ Water is always H2O
law of multiple proportions
elements can combine in different ratios to form different compounds
temperature
-A measure of the average kinetic energy (ke) of particles in a sample of matter
-Is directly proportional to KE
-Does NOT depend on the identity or mass of a substance
-Does NOT depend on the size or mass of a sample
-Not a form of energy and is measured in Celsius, Kelvin, or Fahrenheit
heat
-Thermal energy that flows between objects that are at diff. temperatures
-Transfers through molecular collisions from hotter objects to cooler objects until the two objects are at the same temp.
-Depends on the size of a sample
-Depends on the identity of a substance
-A form of energy that is measured in joules (J)
specific heat capacity
-The amount of heat needed to raise the temp. of 1g of any substance by by 1K or 1 C
-Matter w/a higher specific heat capacity requires more energy to heat
Heat capacity
A matter’s unique capacity to absorb heat
exothermic reaction
q < 0 indicates heat lost
endothermic reaction
q > 0 indicates heat gained
enthalpy
-🔺H can be calculated by using data collected from a chemical reaction that occurs inside a calorimeter
-heat energy released or absorbed by a chemical reaction is equal to the change in enthalpy
Calorimetry
the science of using a calorimeter to measure the change in heat of a physical process or a chemical reaction
conduction (Heat transfer)
energy is transferred by direct contact
radiation (heat transfer)
energy is transferred by electromagnetic radiation through space or air
convection (heat transfer)
energy is transferred by the movement of matter
Sign of a physical change
change in size
Sign of a physical change
change in shape
Sign of a physical change
change in state
Sign of a physical change
new substance is NOT formed
Sign of a chemical change
Change in physical and chemical properties
Sign of a chemical change
formation of a new substance
Sign of a chemical change
color change
Sign of a chemical change
production of heat/light
Sign of a chemical change
formation of gas
Sign of a chemical change
odor change
pure substance
-has a chemical formula
-can NOT be physically separated
mixture
-has NO chemical formula
-can be physically separated
elements
-are pure substances that are made up of only ONE kind of atom
-every symbol on the periodic table represents a pure substance in the form of an element
-an atom is the smallest unit of an element that maintains the properties of that element
compounds
-matter that is composed of atoms of two or more kinds of elements that are chemically bonded
-properties are different than their individual elements (Na + Cl2 = NaCl)
-molecules are the smallest unit of a compound that retains all the properties of that compound
molecules that are not compounds
hydrogen (H2), oxygen (O2), and nitrogen (N2) are examples of molecules that are not compounds because each is composed of atoms of only ONE KIND of element.
Homogenous mixtures
-the composition IS uniform
-different substances that are mixed together cannot be detected but can be physically separated
Homogenous mixtures (solutions)
-made up of very small particles that do not settle
-liquid solutions do not look cloudy
-particles do not scatter light (no Tyndall effect)
homogenous mixtures (alloys)
-solid solutions in which 2 or more metals are uniformly mixed
→ brass (zinc + copper)
→ sterling silver (silver + copper)
heterogenous mixtures
-composition is NOT uniform
-different substances that are mixed together can be detected and be physically separated
Heterogenous mixtures (colloids)
-particles are medium size
-particles do not settle (mayo, cheese, butter, whipped cream)
-particles scatter light (display Tyndall effect)
-separates over time
heterogenous mixtures (suspensions)
-do NOT have uniform composition
-particles are large
-particles settle (orange juice with pulp, muddy water)
7 molecules that are not compounds
hydrogen, nitrogen, oxygen, fluorine, chlorine, bromine, iodine