module 3 - periodic table & energy
1/117
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
118 Terms
What are the properties of F, Cl , Br & I ?
Fluorine - yellow gas at room temp, very reactive and toxic
Chlorine - green gas at room temp, very reactive and toxic
Bromine - orange liquid, very reactive, toxic and often dissolved in water to make a solution
Iodine - grey crystals, reactive, toxic, can go through sublimation and used in solution as an antiseptic
What is the trend in melting/boiling point down Group 7?
As you go down Group 7 melting/boiling point increases due to stronger induced dipole -dipole forces because:
- the number of electrons increases
- the atomic radius increases so there's more points of contact between the molecules so more interaction
How do Group 2 elements react with dilute acids?
X + 2HCl ----> XCl2 + H2 where X is the Group 2 element.
They all react to form salt & bubbles of hydrogen gas and have more vigorous reactions as you react metals further down the group.
More vigorous reactions means more effervescence & the metal dissolves quicker.
How do group 2 oxides react with water?
they release OH- ions and form alkaline solutions of the metal hydroxide
These metal hydroxides are only slightly soluble in water so when the solution becomes saturated any further metal & hydroxide ions form a solid precipitate.
XO + H2O ----> X2+ + 2OH-
How does the solubility of Group 2 metal hydroxides change as you down the group?
The solubility in water increases as you go down the group so the resulting solutions are more alkaline.
- Magnesium hydroxide is only very slightly soluble so has a low hydroxide conc and a pH around 10
- Barium hydroxide however is much more soluble so has a pH around 13
What are Group 2 compounds used for?
Neutralising acidity because they are alkaline.
Calcium Hydroxide is added to fields to increase the pH of acidic soils.
Magnesium and calcium carbonates are often used as antacids for treating acid indigestion. A suspension of magnesium hydroxide is also used.
How does the 1st ionisation energy change across a period?
First ionisation energy increases:
- nuclear charge increases
- similar shielding
- nuclear attraction increases
- atomic radius decreases
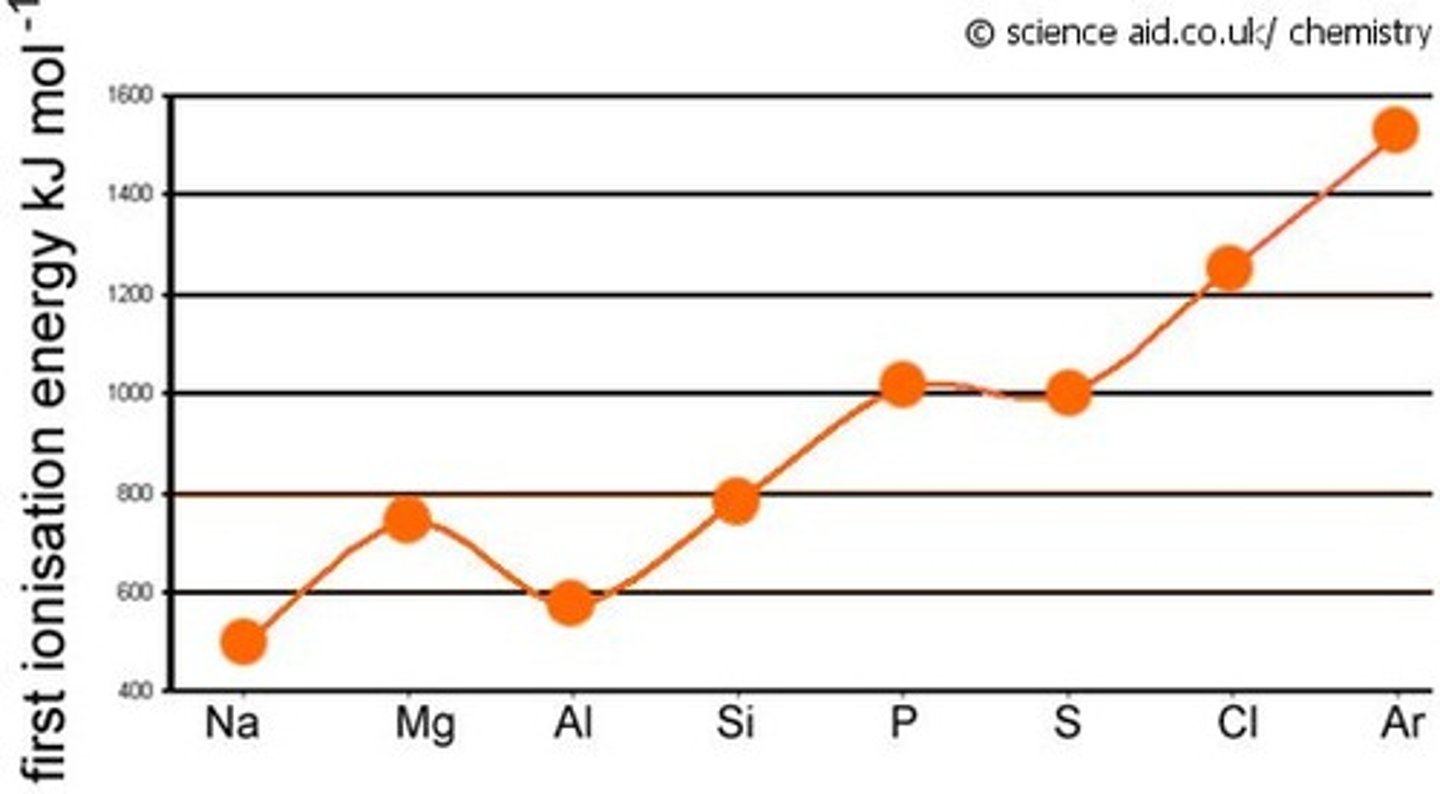
What is the trend in first ionisation energy across a period?
As you go across a period the first ionisation energy increases because :
- atomic radius decreases
- electron shielding stays the same
- nuclear charge increases/ increase in number of protons
This means the the nuclear attraction increases so more energy is required to remove the outer shell electron
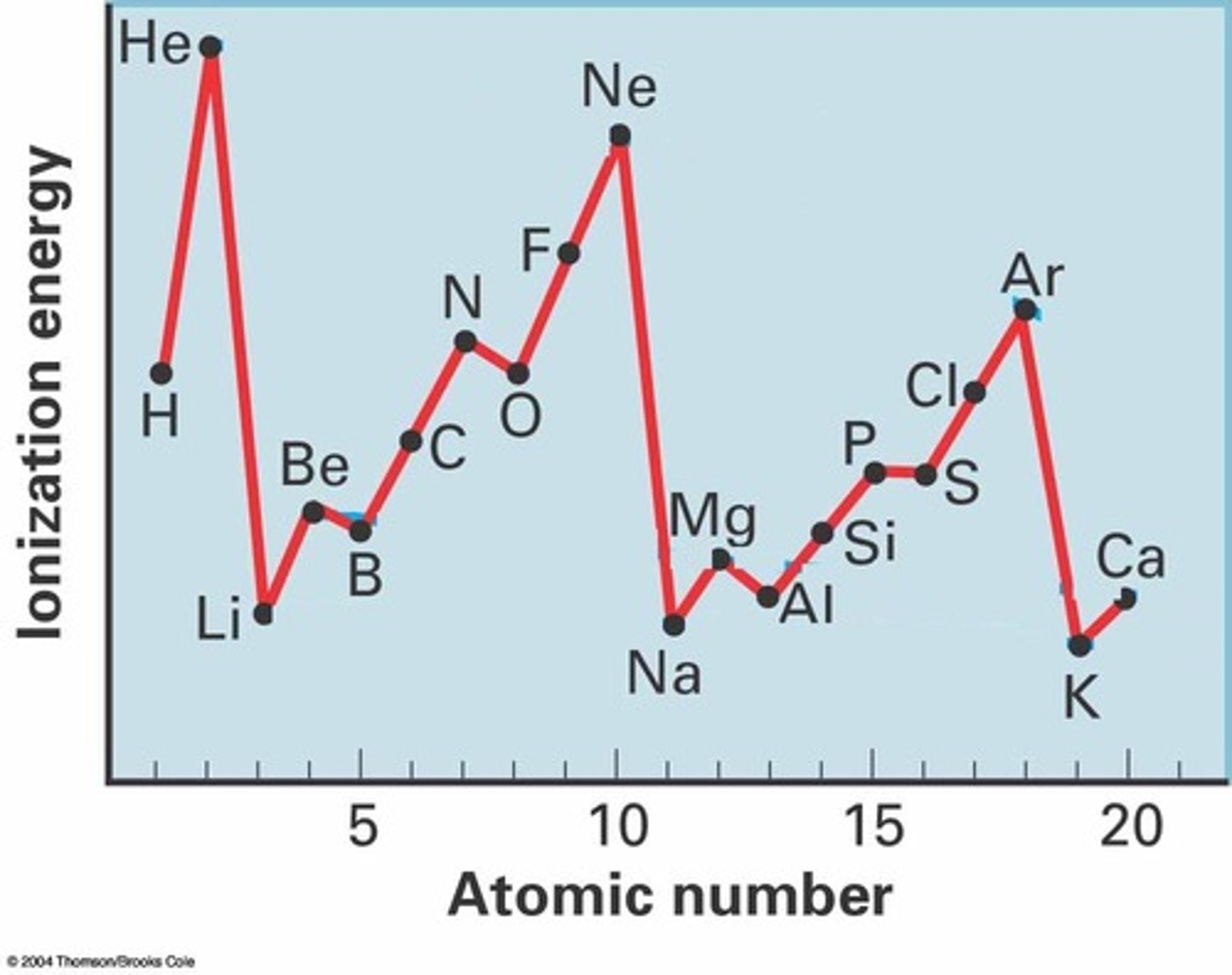
Why is there a dip in ionistation energy across a period between Group 5 & 6?
There is a dip because the Group 5 element has only 1 electrons in the p subshell whereas the Group 6 element has 2 electrons/a pair of electrons in the p subshell.
Electrons repel each other in a pair so its easier to remove electrons from Group 6 because of the added electron.
What is the trend in melting point down group 2?
Decreases due to larger atomic radii down group. The delocalised electrons experience less attraction to nucleus, so bonding is weaker, melting point is lower
How do the alkaline metals react with oxygen?
They react with oxygen to form a metal oxide e.g. MgO which is white and burn with a bright white light for Magnesium.
Why are Group 2 elements called reducing agents when they go through redox reaction?
They are oxidised so they lose 2 electrons which can be gained by another atom etc.
How do Group 2 elements react with water?
X + 2H2O ⟶ X(OH)2 + H2 Where X is the Group 2 element.
Water and magnesium react very slowly but the reaction becomes more vigorous with metals further down the group and appear as bubbles of hydrogen.
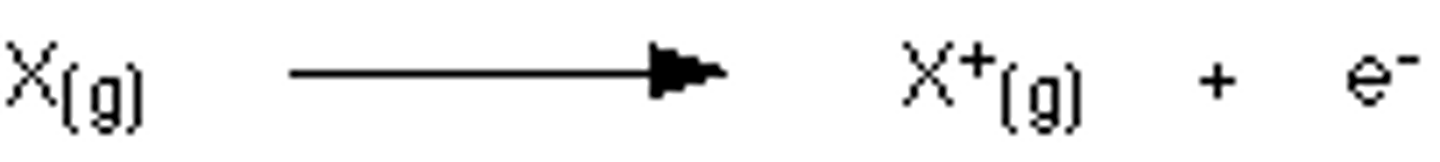
What is ionisation in terms of ionisation energy?
When an atom loses an electron from its outer shell only
What is the 1st ionisation energy?
The energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions/ energy required to remove electrons
What is ionisation energy measured in?
kJmol-¹ . the values can tell us a lot about electron configuration
What are the factors which effect ionisation energy?
atomic radius, nuclear charge, electron shielding
How does the atomic radius effect ionisation energy?
the bigger the atom is the further the outer electron is from the nucleus so there's less attraction between the two making the electron easier to lose lowering the IE
How does the nuclear charge effect the ionisation energy?
the more protons there are in the nucleus the more attraction there is to the outer electron increasing ionisation energy : this is why the 2nd 1E is higher than the 1st
How does electron shielding effect ionisation energy?
the inner shells of an atom repel the outer shells the more inner shells there are the more repulsion there is reducing the attraction between nucleus and outer electron
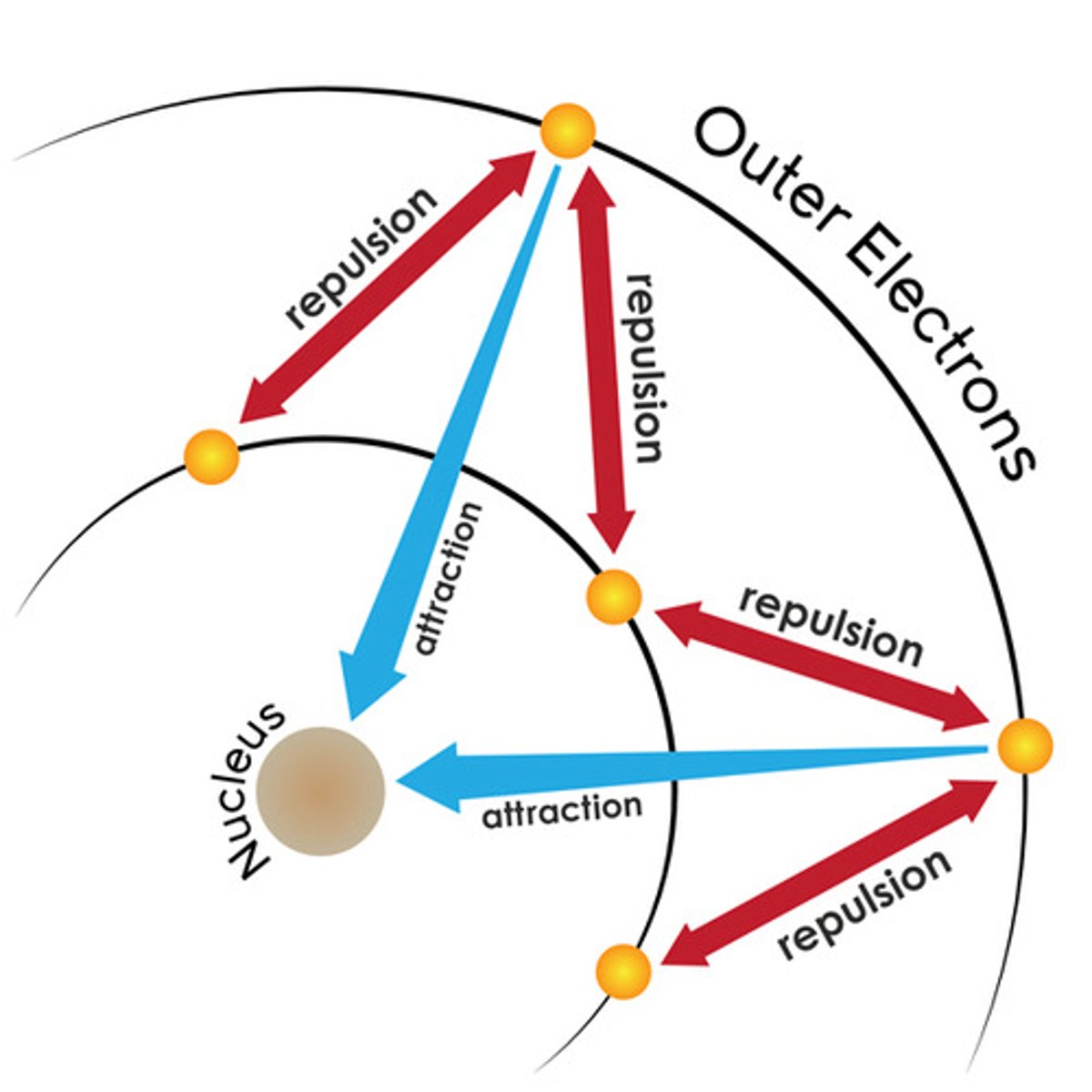
How do periodic trends in first IEs support the existence of shells and sub-shells?
- there is a general increase in first IE across each period
- there is a sharp decrease in first IE between the end of one period and the start of the next - start of new shell or subshell
How do first IEs change when going down a group?
There is a general decrease in ionisation despite the increase in protons and so nuclear attraction increasing because of :
- the increase in atomic radius making the outer electron easier to lose
- increase in electron shielding due to greater number of shells leading to more repulsion
These combined outweigh the attraction due to nuclear charge.
How would you find identify an atom from its successive ionisation energies?
- Find where the biggest jump in ionisation energies is between e.g. between 9 & 10
- This shows that the 10th electron must be being removed from a different shell closer to the nucleus
- so we can use this to find how many electrons are in each shell as we know 2 in first shell etc
How would you identify what group an atom is in (and then its identity) from its period and successive ionisation energies?
- Find where the large increase in ionisation energy is e.g between 3rd & 4th
- Therefore there must be 3 electrons in the outer shell and so its in group 3
- Then use both the group and period its in to identify the atom
Why is there a decrease in first ionisation energy between Beryillium and Boron?
The electrons in the 2p orbital have a slightly higher energy than the 2s orbitals making them more easily lost.
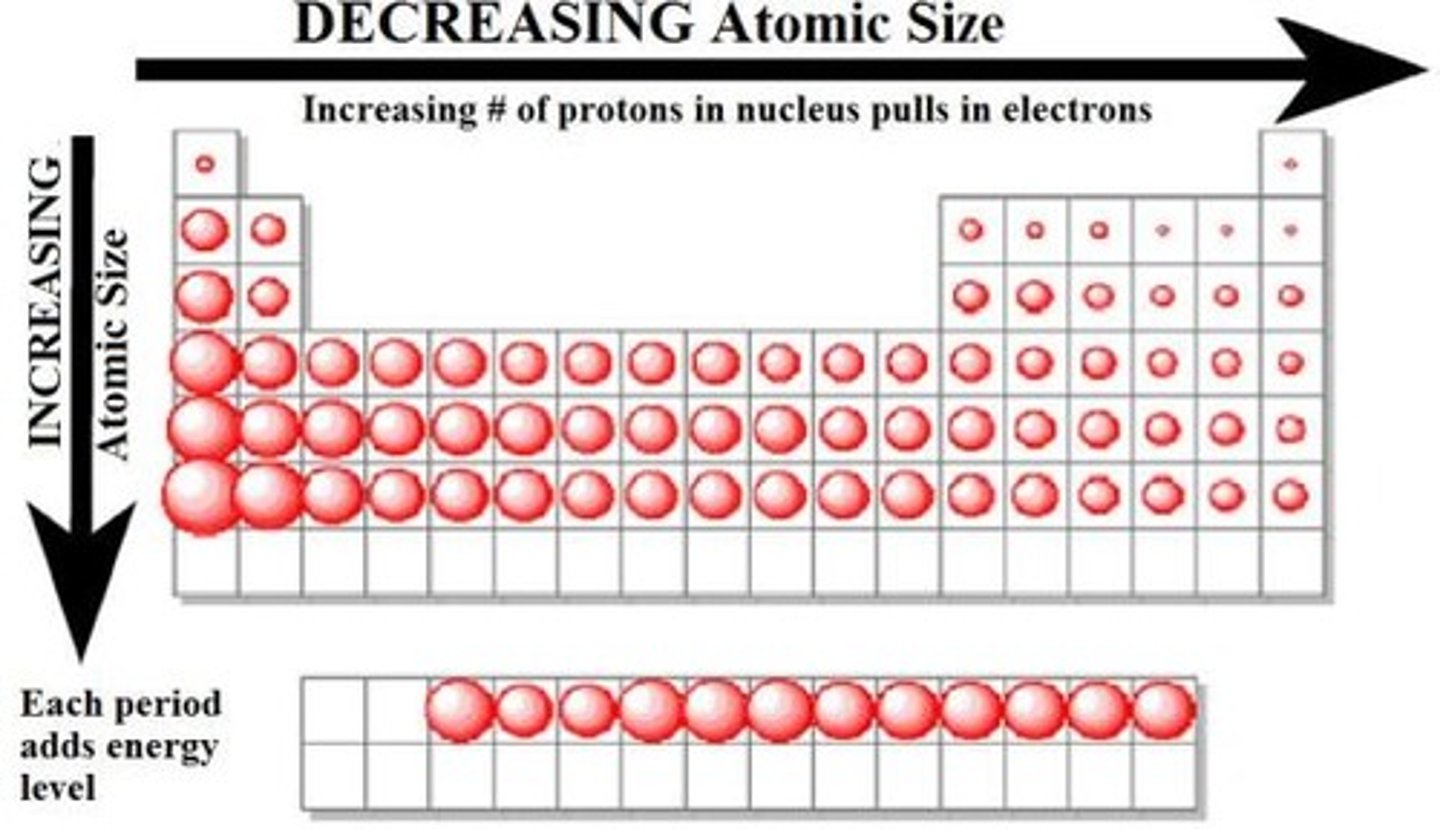
What is the trend in atomic radius across a period?
As you go across a period the atomic radius decreases because:
- the outer electrons stay in the same shell
- number of protons/ nuclear charge increases
- electron shielding stays the same
This means there's a stronger attraction between the nucleus and outer shell electrons so they're pulled closer to the nucleus= smaller radii
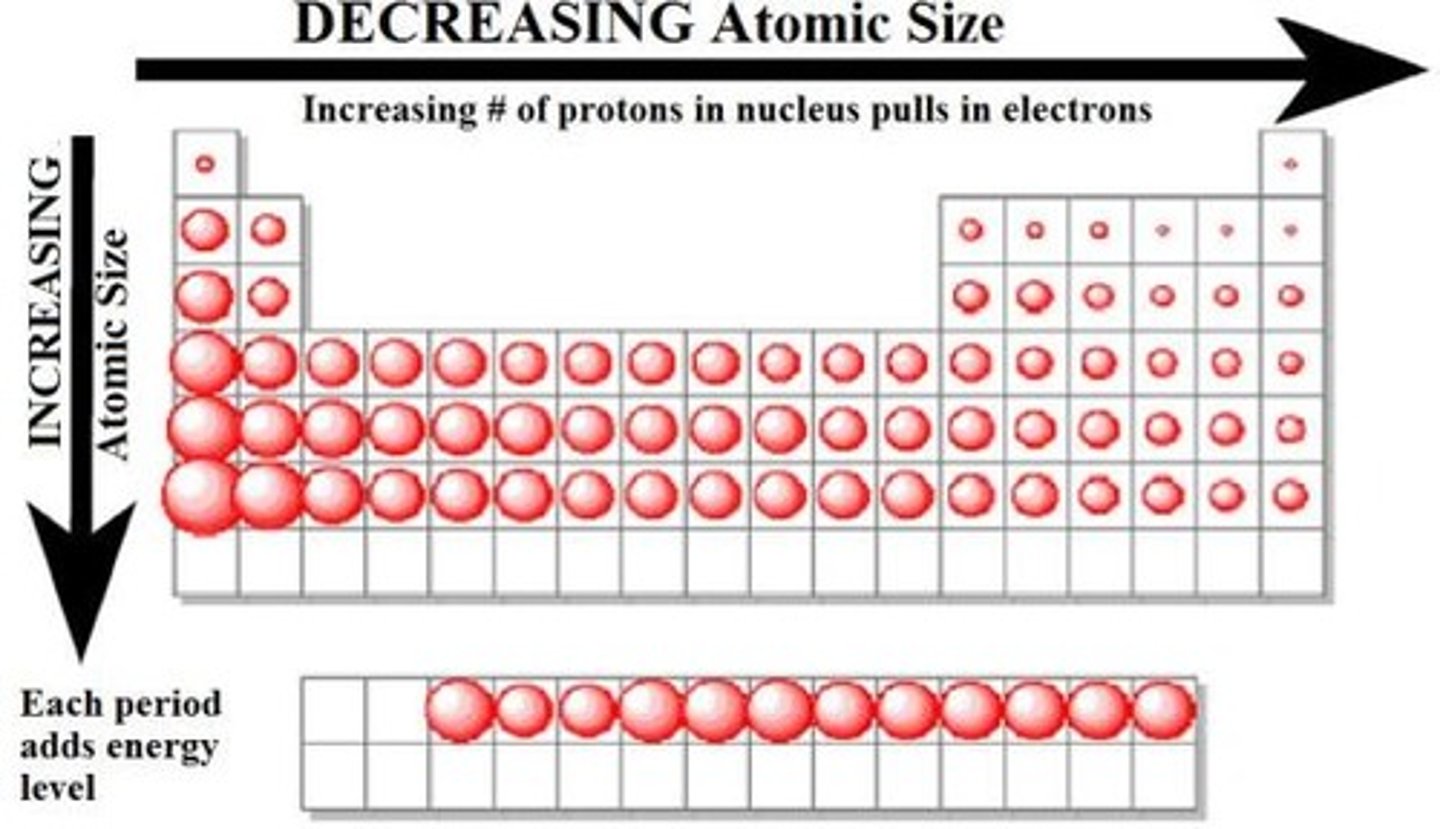
Why is there a dip in ionistation energy across a period between Group 2 & 3?
There is a dip because the Group 2 element has its electrons only in the 2s subshell and the Group 3 element has at least one electron in the 2p subshell.
The 2p subshell (and all p subshells) has more energy than the 2s subshell so less energy is required to remove an electron from the 2p subshell than the 2s subshell.
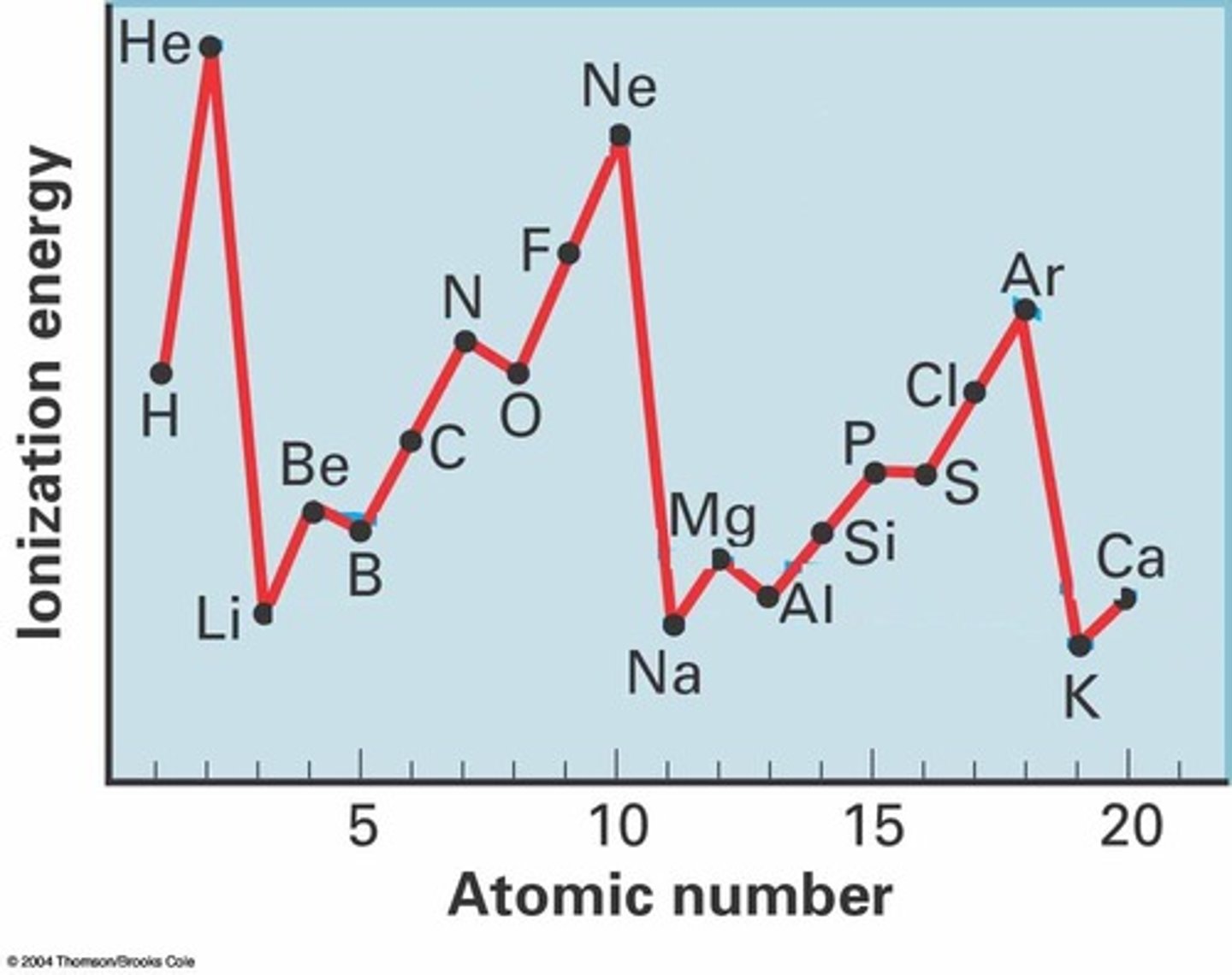
What is the trend in electronegativity across a period?
As you go across the periodic table electronegativity increases because:
- atomic radius decreases
- nuclear charge increases
- number of shells & so electron shielding remain the same
This means the nuclear attraction increases so there's a stronger ability to attract a bonding pair of electrons.
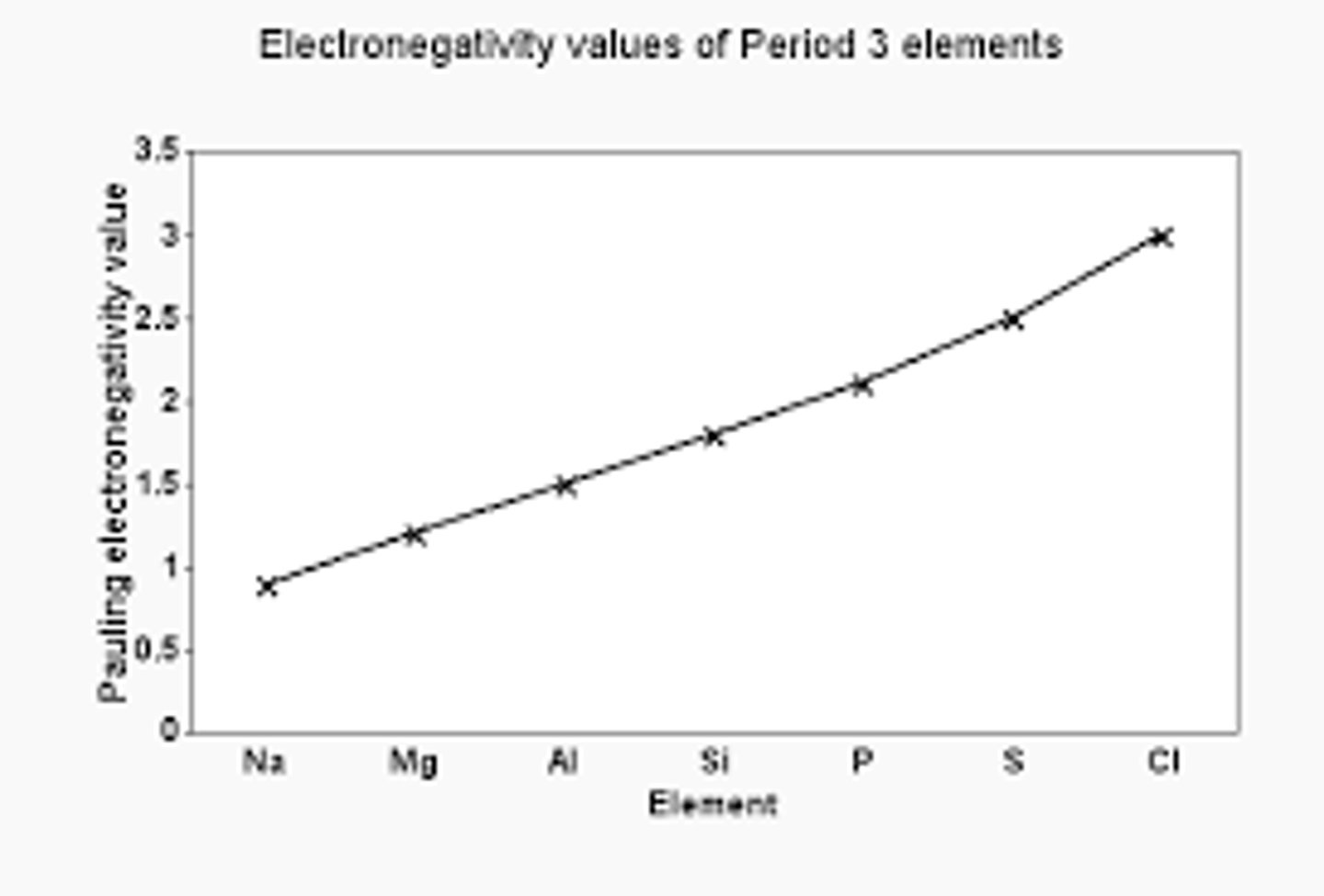
What is the general trend in boiling/melting point across a period?
There is a general decrease in melting/boiling point across a period because:
- the type of element changes from metal to non-metal
- so the bonding changes from strong metallic bonding to weak intermolecular forces between the atoms/molecules
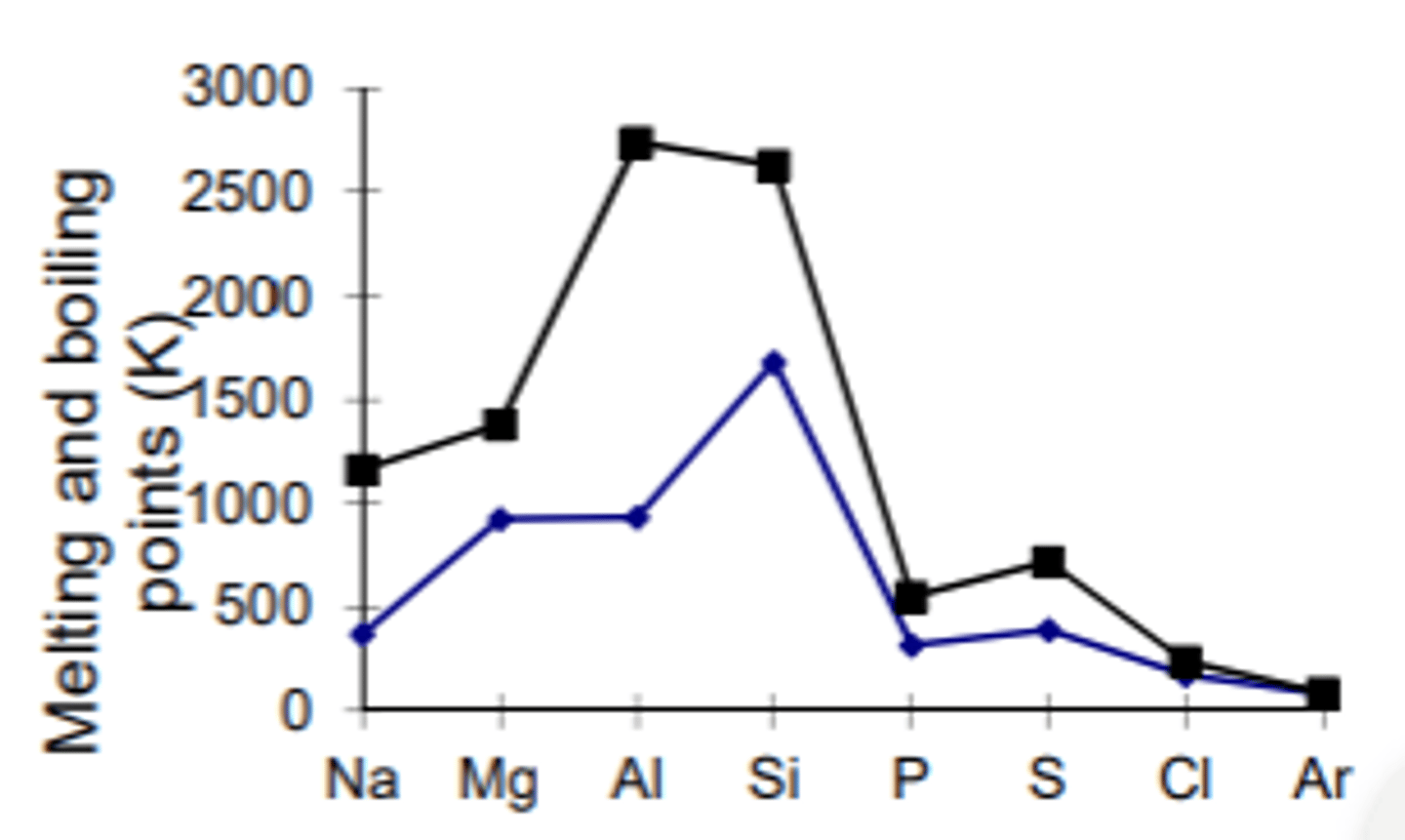
Why is there an increase in boiling point between the metals as you go across a period e.g. between Na & Al in period 3?
The number of electrons increases charge density increase while atomic radius deceases so the metallic bonds are stronger in e.g. Al vs Na so they require more energy to be overcome/broken.
What is the trend in atomic radius down Group 7?
As you go down atomic radius increases because of the increase in number of electrons and so shells.
What is the trend in electronegativity down Group 7?
As the halogen atoms get bigger, any bonding pair of electrons gets further and further away from the halogen nucleus, and so is less strongly attracted towards it. In other words, as you go down the Group, the elements become less electronegative.
What is the trend in first ionisation energy down Group 7?
As you go down Group 7 first ionisation decreases because:
- atomic radius increases
- electron shielding increases
The combined effect of this outweigh the increase in nuclear charge so there is a decrease in nuclear attraction.
What happens when some Group 2 metals are reacted with Sulfuric acid?
An insoluble sulfate is formed and the insolubility of the sulfate formed increases as you go down Group 2.
E.g. Calcium sulfate can be soluble but Strontium sulfate is completely insoluble.
Why are halogens called oxidising agents in redox reactions?
In a redox reaction the halogen atoms are reduced and gain electrons to form halide ions.
Another reactant in the reaction loses it’s electrons to the halogen atoms for this to happen - it is oxidised.
The halogen is an oxidising agent because it has oxidised another species.
How can halogen-halide displacement reactions be used to determine the reactivity of the halogens?
If a reaction takes place in a halogen-halide displacement reaction then the halogen has displaced the halide and the solution will change colour.
Repeating the reactions with all the halogens shows that the reactivity of halogens decreases as you go down the group.
How is a halogen-halide displacement reaction done?
A solution of a halogen is added to aqueous solutions of another halide which is in an ionic compound.
E.g. a solution of Cl2 is added to an aqueous solution of KBr where there are Br- ions.
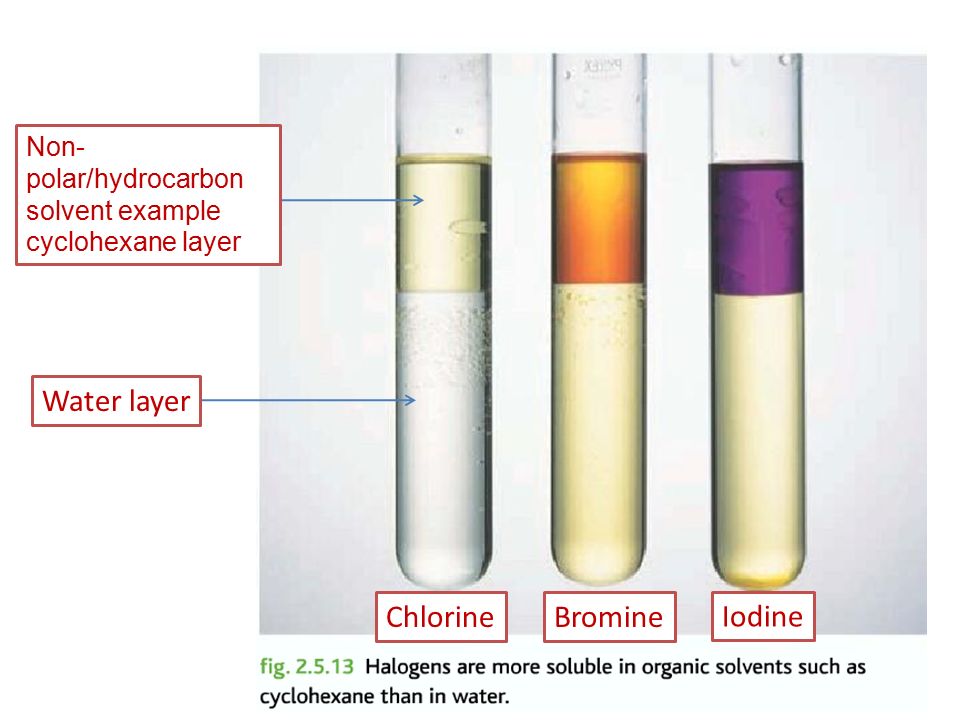
What is the colour of these halogen solutions in water?
Cl2 - pale green
Br2 - orange
I2 - brown

Why is cyclohexane sometimes added to halogen solutions?
Solutions of Iodine & Bromine can be very had to tell apart so an organic non-polar solvent like cylcohexane are added.
The non-polar halogens dissolve more readily in cyclohexane than in water so the colours are stronger and easier to tell apart.
What is the colour of the halogen solutions in water when cyclohexane is added?
The solution in cyclohexane is the top layer/ organic layer.
Cl2 - pale green
Br2 - orange
I2 - violet/purple
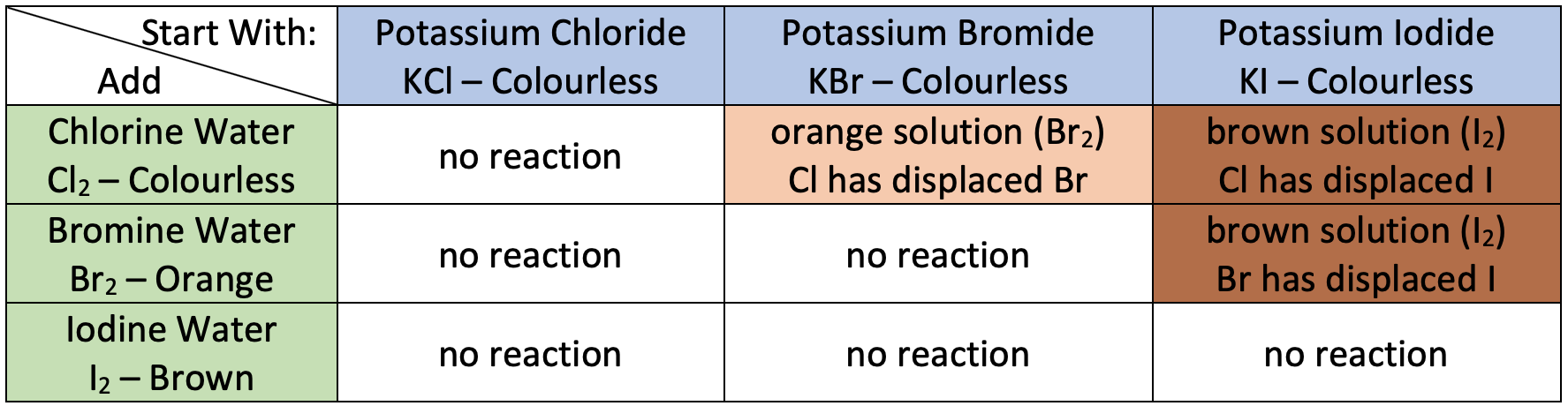
What are the results from the halogen-halide displacement reactions?
Chlorine reacts with both Bromide & Iodide.
Bromine reacts with Iodide only.
Iodine hasn’t reacted with either Bromide or Chloride.
What is happening in a halogen-halide displacement reaction?
If the halogen is more reactive it will kick out the halide ion out of the compound it’s in because the cation is more attracted to the halogen than the halide it was originally bonded to:
Cl2 + 2NaBr ——> 2NaCl + Br2
Or as an ionic equation : Cl2 + 2Br- ——> 2Cl- + Br2
Chlorine is reduced & Bromide is oxidised.
What is disproportionation?
Disproportionation is a redox reaction where the same atom/element is both reduced and oxidised.
E.g. Chlorine + water
Chlorine + Dilute Sodium Hydroxide
What is the disproportionation reaction between water & chlorine?
Cl2 + H2O ——> HClO + HCl
Chlorine —→ Chloric acid = oxidation
Chlorine ——> Hydrocholric acid = reduction
Why is the disproportionation reaction between water & chlorine important?
The products of it are 2 acids which can kill bacteria and act as a weak bleach.
This is why chlorine is added to water to act as a disinfectant.
What is the reaction of chlorine with cold, dilute aqueous sodium hydroxide?
Cl2 + 2NaOH ——> NaClO + NaCl + H2O
The resulting solution contains lots of Chlorate ions from the Sodium Chlorate and so can be used as household bleach.
How can a silver nitrate test be used to identify which halide is present in a solution?
The aqueous silver nitrate solution AgNO3 is added to an aqueous halide solution.
The silver halide precipitates formed are different colours depending on which halide it is :
Silver chloride = white
Silver bromide = cream
Silver Iodide = yellow
Silver Floride is soluble so no precipitate is formed.
Why are the silver halides formed then reacted with aqueous ammonia?
The colours of the precipitate can be hard to tell apart.
When reacted with ammonia:
AgCl - precipitate dissolves to give a colourless solution
AgBr - precipitate is almost unchanged using dilute ammonia solution, but dissolves in concentrated ammonia solution to give a colourless solution
AgI - precipitate is insoluble in ammonia solution of any concentration
What is enthalpy?
The measure of the heat energy in a chemical system - it’s sometimes thought as the energy stored within bonds.
Enthalpy itself cannot be measured but enthalpy changes can.
Represented as H.
What is an enthalpy change?
The difference in enthalpies between the products & reactants in a reaction.
ΔH = H(products) - H(reactants)
What is the conservation of energy?
A fundamental rule of science stating that energy cannot be created or destroyed.
In an enthalpy change this means no energy is lost in a system its instead transferred to the surroundings.
What are the anions that you can test for?
Carbonate ions
Sulfate ions
Halide ions
What is the test for carbonate ions?
React the sample with dilute nitric acid to form carbon dioxide gas.
This would be observed as bubbles in the sample
We can prove the gas is carbon dixoxide by bubbling the gas through lime water - saturated aqueous solution of calcium hydroxide
The carbon dioxide will react forming a a fine white precipitate turning the lime water cloudy
What is the test for sulfate ions?
We can use the reaction of barium and a sulfate ion containing solution as a test as most sulfates are soluble in water but barium sulfate isn’t making it easy to observe.
The test involves reacting the sample and barium nitrate together to form a white precipitate - barium sulfate.
*usually the barium ions are part of a halide compound but if you’re also testing for halides then barium nitrate will be used.
What is the test for halide ions?
Reaction between a halide and silver forming a silver halide precipitate.
Silver is used as most halide compounds are soluble in water but silver halides aren’t making them easy to observe
In which order should the tests for anions be done in and why?
Carbonate —> Sulfate —> Halide
Carbonate is first as in the test you react with a dilute acid to produce effervescence, neither halide nor sulfate ions will produce a gas so doing this first rules them out if a positive result occurs
Sulfate test is after the carbonate one as barium carbonate also produced a white insoluble precipitate like barium sulfate so you have to rule out carbonate as an option as you won’t know which of the 2 your sample is
Halide tests have to be done last as silver carbonate and silver sulfate are both insoluble precipitates like the silver halides so we need to have ruled out both possibilities before hand to identify the sample
What order of reactions should you go in to analyse a mixture of chemicals with a mixture of anions?
Still do - Carbonate → Sulfate → Halide
But :
Carry out the tests on the same solution
Use dilute nitric acid only for the carbonate test as sulfuric acid contains sulfate ions & HCl contains chloride ions which will interfere with the sulfate & halide tests
Don’t use barium chloride in the sulfate test as the chloride ions will show up in the halide test
What is the cation you can test for and how do you test for it?
Test for ammonium ions NH4+.
Heat aqueous ammonium ions and aqueous hydroxide ions to form ammonia gas.
Add aqueous sodium hydroxide to a solution of an ammonium ion containing compound
Ammonia gas is produced - but no bubbles will be seen
The misture is warmed relasing ammonia gas
You can test the gas with moist pH indicator paper - ammonia is alkaline so the paper will go blue
What is the difference between exothermic and endothermic reactions?
Exothermic - energy is transferred from the system to the surrounding - enthalpy change is negative
Endothermic - energy is transferred from the surroundings to the system - enthalpy change is positive
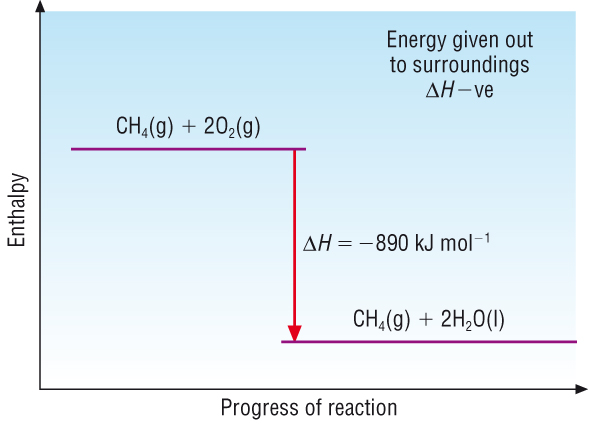
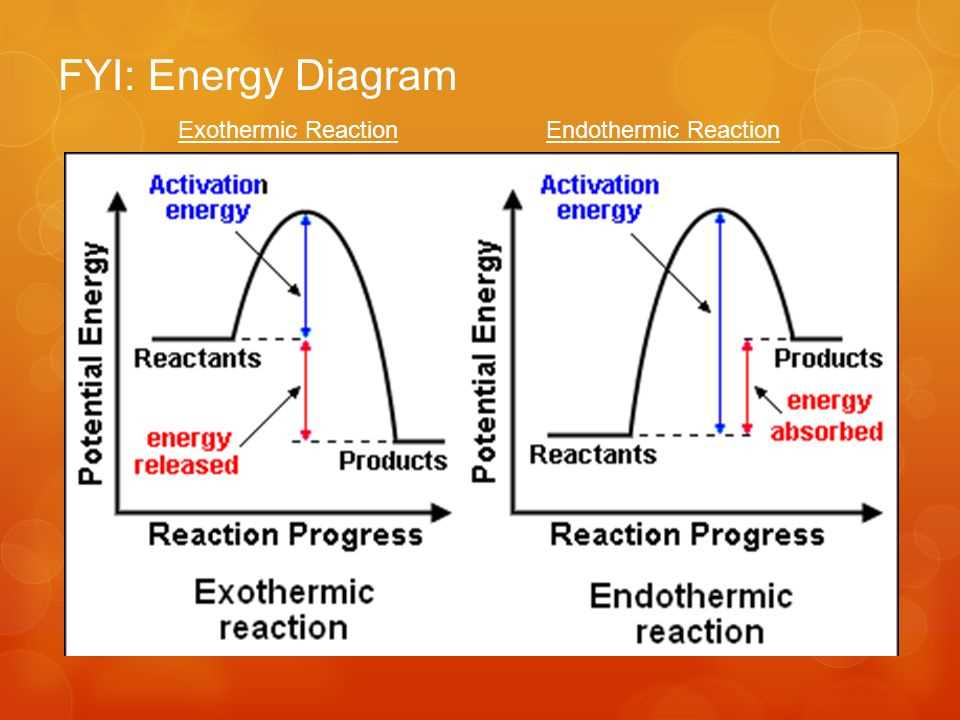
What is the enthalpy profile diagram for an exothermic reaction and what does it show?
The chemical system releases heat energy to the surroundings
Any energy loss by the chemical system is balanced by the same energy gain by the surroundings
Enthalpy change is negative
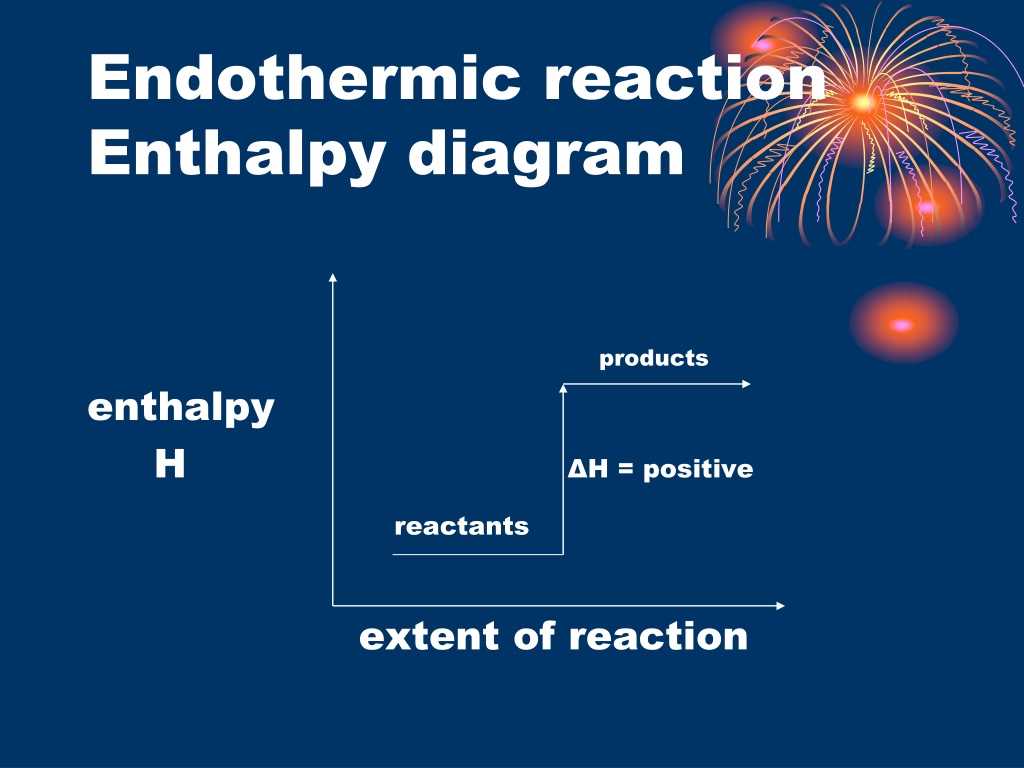
What is the enthalpy profile diagram for an endothermic reaction and what does it show?
The chemical system takes in energy from the surroundings
Any energy gain by the chemical system is balanced by the same energy loss by the surroundings
Enthalpy change is positive
What is activation energy?
The energy input required to break bonds between the reactants so that they can reform to make the products.
Activation energy is the minimum energy required for a reaction to take place.
In general, reactions with small activation energies take place very rapidly as the energy required is readily available in the surroundings.
Why are enthalpy changes measured under standard conditions?
The enthalpy change for a reaction can vary slightly depending on the conditions used - using standard conditions means that all enthalpy changes can be compared etc.
What does standard conditions ⦵ mean?
Pressure is 100kPa
Temperature is 298K
Concentrations for solutions are 1mol/dm3
The standard state is the physical state of a substance in these conditions e.g. water would be a liquid
What are all the different types of enthalpy changes to remember?
Standard enthalpy change of reaction
Standard enthalpy change of formation
Standard enthalpy change of combustion
Standard enthalpy change of neutralisation
What is the standard enthalpy change of reaction?
The enthalpy change that accompanies a reaction in the molar qunatities expressed in a chemical equation under standard conditions.
What is the standard enthalpy change of formation?
The enthalpy change when one mole of a compound is formed from its substituent elements under standard conditions.
E.g. Mg + ½ O2 —> MgO enthalpy change = -602
The standard enthalpy change of formation for an element is 0 for all elements as we end and start with the same thing so there’s no difference in enthalpy.
What is the standard enthalpy change of combustion?
The enthalpy change when one mole of a substance reacts completely with oxygen under standard conditions.
Only complete combustion occurs.
E.g. C4H10 + 6 ½ O2 —> 4CO2 + 5H2O
Enthalpy change = -2877 / will always be negative as it’s an exothermic reaction always.
What is the standard enthalpy change of neutralisation?
The enthalpy change when an acid and a base react together to form one mole of water under standard condition.
HCl + NaOH —> H2O + NaCl Enthalpy change = -57
The value of all standard enthalpy changes of neutralisation is the same as the reaction of H+ and OH- to form water is the same in all of them.
What is enthalpy change measured in?
kJmol-1
How can you calculate an energy change of the surroundings?
q = mcΔT
q = heat energy
m = mass of the surroundings
c = specific heat capacity of the surroundings
ΔT = change in temperature
What is specific heat capacity?
The amount of energy required to raise the temperature of 1g of a substance by 1K.
What is the specific heat capacity of water and why do we need to use it?
c = 4.18 Jg-1K-1
For most experiments where we measure the energy change we’re measuring the temp change of water or aqueous solutions / water acts as the surroundings.
Why might an experimental enthalpy change of combustion be inaccurate?
The data book values are obtained using much more sophisticated apparatus so there isn’t the same loss of heat to elsewhere/not the water.
Heat lost to the surroundings other than the water e.g. the beaker & the air surrounding the flame
Incomplete combustion
Evaporation of the fuel/substance from the wick - spirit burners usually have a lid in order to prevent this but in an experiment it’s important for the burner to be weighed as soon as extinguishing the flame
Non-standard conditions
All but the non-standard condition would lead to a value that’s less exothermic than expected.
What is an average bond enthalpy?
The energy required to break one mole of a specified type of bond in a gaseous molecule.
The actual bond enthalpy can vary depending on the chemical environment of the bond e.g. compound and the average calculated from these can be different from the specific bond enthalpy in a specific compound.
Why are bond enthalpies always positive?
Energy is always required to break bonds so they are always endothermic.
How can we use average bond enthalpies?
We can use them to calculate the enthalpy changes of a reaction without carrying out any experiments.
What is the difference bewteen bond breaking and bond making?
Bond breaking requires energy so is endothermic.
Bond making releases energy so is exothermic.
What can the difference between the energy required for bond breaking vs bond making show about a reaction?
Energy required to break bond > energy released from bond making
—> Endothermic reaction
Energy required to break bond < energy released from bond making
—> Exothermic reaction
What is the equation for ΔrH from average bond enthalpies?
ΔrH = sum of reactants - sum of products
What is Hess’s Law?
If a reaction can take place by 2(or more) routes and the starting and finishing conditions are the same the total enthalpy change is the for each route.
Aka. the enthalpy change for a reaction will be the same no matter which reaction pathway you chose to go from the reactants to the products as long as both are the same.
What does Hess’s Law allow us to do?
It allows us to find the enthalpy change of reactions that are very difficult do determine directly through a physical experiement - calorimetry.
It can be used for any number of enthalpy changes provided that there’s only one unknown enthalpy change.
What are the 2 indirect ways we can get an unknown enthalpy change from a Hess’s cycle?
Enthalpy changes from ΔfH of the reactants and products as they are both formed from the same element
Enthalpy changes from ΔCH of the combustion of the products and reactants are because they are made from the same elements they will combust to form the same combustion products
What does a Hess’s cycle for an enthalpy change using ΔfH look like?
In formation the arrows from the shared elements go upwards to the reactants and products
Use the vectors principles to go from the reactants to products but using the enthalpy changes of formation or ΔHr = ΔHreactants – ΔHproducts
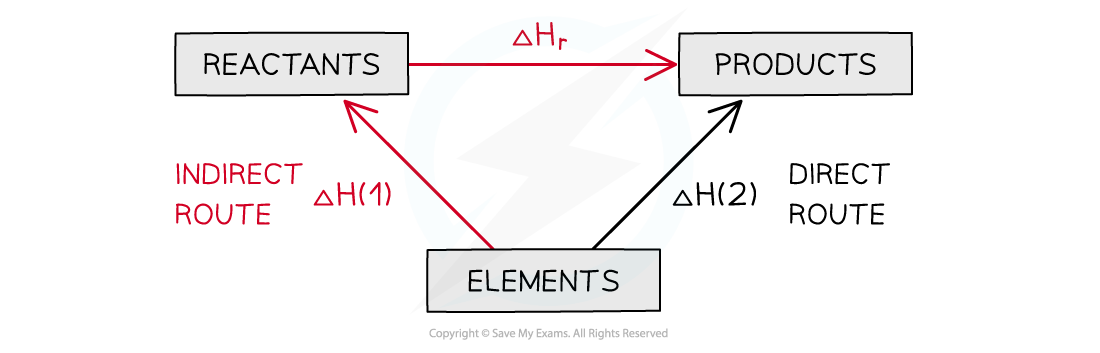
What does a Hess’s cycle for an enthalpy change using ΔcH look like?
In combustion the arrows from the shared elements go towards them from the reactants & products
Use the vectors principles to go from the reactants to products but using the enthalpy change of combustion or ΔHr = ΔHreactants – ΔHproducts
What are the equations to find an enthalpy change from :
average bond enthalpies
enthalpies of formation
enthalpies of combustion?
Average bond enthalpies - ΔHr = ΔHreactants – ΔHproducts
Enthalpies of formation - ΔHr = ΔHproducts – ΔHreactants
Enthalpies of combustion - ΔHr = ΔHreactants – ΔHproducts
What is dynamic equilibrium/an equilibrium system?
When the rate of the forwards reaction is the same as the rate of the backwards reaction so the concentrations of the reactants and products do not change.
A dynamic equilibrium also only takes place in a closed system.
An equilibrium system is one which is dynamic so they have the. same definition
What is a closed system?
A chemical system where the system doesn’t interact with it’s surroundings so energy etc is transferred between them.
The temp, pressure & concs of the reactants and products are unaffected by outside influences.
What is the position of equilibrium?
The position of equilibrium indicates the extent of the reaction.
In a reversible reaction the position of equilibrium can change when the conditions of the reaction change.
What is le Chatelier’s Principle?
le Chatelier’s Principle states that when a system in equilibrium is subjected to an external change the system readjusts itself to minimise the effect of the change.
The system will always counteract any change.
What are the conditions that can effect equilibrium according to le Chatelier’s Principles?
Concentration
Temperature
Pressure - if gases are involved
How does concentration effect equilibrium according to le Chatelier’s Principles?
Changing the concentration of a reactant or product in an equilibrium system will change the rate of the forward or reverse reaction. This changes the eqm position.
The system will then counteract this change by shifting the eqm position towards the opposite reaction/the unchanged one.
How can adding H+ ions or OH- ions effect certain reactions at equilibrium?
For some reversible reaction which are effected by the concentration of H+ or OH- ions e.g. the equilibrium between CrO42- ←→Cr2O72- - this is due to a sensitivity to changes in acid conc.
Adding acid/H+ to the reaction means we have too many reactant particles do eqm position shifts to the right turning the solution yellow & vice versa with the OH- ions.
How does temperature effect equilibrium according to le Chatelier’s Principles?
A change in temperature will result in the eqm position changing.
If the temperature is increased the eqm position will move towards the endothermic reaction.
If the temperature is decreased the eqm position will move towards the exothermic reaction
How does pressure effect equilibrium according to le Chatelier’s Principles?
Changes in pressure of a system containing gases in equilibrium may result in the eqm position changing but only of there is already an uneven amount of gaseous molecules on either side.
Increasing the pressure of a dynamic equilibrium will shift the eqm position to the side with fewer molecules in order to reduce the pressure.
Decreasing the pressure will shift the eqm position to the side with more molecules in order to counteract the change.
How does adding a catalyst effect a dynamic equilibrium?
A catalyst doesn’t change the position of equilibrium - it only speeds up the rates of the forward and reverse reaction but each an equal amount.
A catalyst will increase the rate at which an equilibrium is established - the eqm is reached faster.
How does a catalyst work?
A catalyst provides an alternate pathway for a reaction which has a lower activation energy so increases the rate of reaction.
What is KC?
Equilibrium constant in terms of concentration.
What can we use KC for?
It shows us the exact position of equilibrium for a specific reaction.