Chemistry: Topic 2: Bonding, structure, and the properties of matter
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
110 Terms
What are the three types of strong chemical bonds?
Ionic
Covalent
Metallic
What are the particles like in ionic bonding?
Oppositely charged ions
What are the particles like in covalent bonding?
Atoms which share pairs of electrons
What are the particles like in metallic bonding?
Atoms which share delocalised electrons
What does ionic bonding occur in?
Compounds formed from metals combined with non-metals
What does covalent bonding occur in?
Compounds of non-metals
What does metallic bonding occur in?
Metallic elements and alloys
What is an ion?
A charged particle
What type of ions do groups 1 and 2 form?
positive (cations)
What type of ions do groups 6 and 7 form?
negative (anions)
Give the ions formed by elements in each group
Group 1 = 1+ ion
Group 2 = 2+ ion
Group 3 = 3+ ion
Group 4 = 4+or 4- ion
Group 5 = 3- ion
Group 6 = 2- ion
Group 7 = 1- ion
What happens when a metal atom reacts with a non-metal atom?
Electrons in the outer shell of the metal atom are transferred
Describe how an ionic bond forms
The metal atom loses electrons to become a positively charged ion
The non-metal atom gains these electrons to become a negatively charged ion
They have opposite charges to they are strongly attracted to one another by electronic forces
What bonding is there in sodium chloride (NaCl)?
Ionic
Describe the ionic bonding of sodium chloride
The sodium atom gives up its outer electron, becoming an Na+ ion
The chlorine atom picks up the electron becoming a Cl- ion
The Na+ and Cl- ions are attracted together as they have opposite charges
What are the ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 like?
They have the electronic structure of a noble gas (full outer shells)
What does the charge on the ions produced by metals in Groups 1 and 2 and by non-metals in Groups 6 and 7 relate to?
The group number of the element in the periodic table
Describe how Potassium forms an ionic compound with an ion of sulfur
electrons transferred from potassium to sulfur
two potassium atoms each lose one electron
forming K+ / 1+ ions
sulfur atoms gain 2 electrons
forming S2− / 2− ions
How can the electron transfer during the formation of an ionic compound be represented?
By a dot and cross diagram
Why are dot and cross diagrams useful?
show the arrangement of electrons
shows where each electron came from
Why are dot and cross diagrams not useful?
they don’t show the structure of the compound
don’t show the size of the ions
don’t show how they’re arranged
What structure do ionic compounds have?
Regular giant ionic lattice structures
What are ionic compounds held togther by?
Strong electrostatic forces of attraction between oppositely charged ions
These forces act in all directions in the lattice
What is the 3D balls model of ionic compounds like?
Shows the relative sizes of the atoms
Shows the regular pattern of an ionic crystal
Only lets you see the outer layer of the compound
What is the ball and stick model of ionic compounds like?
Shows regular pattern of an ionic crystal
Shows how all ions are arranged
Suggests the compound extends beyond what is shown
Isn’t to scale, so the relative sizes of the atoms may not be shown
In reality, there are no gaps/ visible bonds between the ions
What are the properties of ionic compounds?
High MP and BP
Can’t conduct electricity when solid, but can when molten or aqueous
Why do ionic compounds have high MP and BPs?
There are many strong bonds between the ions
It takes a large amounts of energy needed to break the many strong bonds
Why can ionic compounds not conduct electricity when solid?
The ions are held in place, so electrons cannot move to carry an electric charge
Why can ionic compounds conduct electricity when molten or aqueous?
When melted, the ions are free to move so a charge can flow
When dissolved, the ions separate and are free to move in the solution, letting them carry an electric charge
How do you work out the empirical formula of an ionic compound from a diagram?
Dot and cross diagram - count how many atoms there are of each element
3D diagrams - work out the ions and charges, then balance them so the overall charge of the compound is 0
What are the complex molecular cations?
Ammonium - NH4+
What are the complex molecular anions?
Hydroxide - OH-
Nitrate - NO3-
Carbonate - CO32-
Sulfate - SO42-
How does a covalent bond form?
When two non-metal atoms share pairs of covalent bonds
Why are covalent bonds so strong?
The positively charged nuclei of the bonded atoms are attracted to the shared pair by electrostatic forces
What might covalently bonded substances consist of?
Small molecules
Which electrons do atoms share in covalent bonds?
Those on the outer shell (as this is where the highest energy levels are)
How many extra electrons does each single covalent bond provide for each atom
1
Why do atoms bond covalently?
To get a full outer shell, which gives it a stable structure
Dot and cross diagrams for covalent bonds:
What do they look like?
Why are/ aren’t they useful?
Only draw outer shell
Electrons drawn in the overlap between the shells are shared
Useful for showing which atoms in a covalent bond come from
Don’t show the relative sizes of the atoms
Don’t show how the atoms are arranged in space
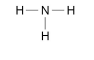
Displayed formula diagrams for covalent bonds:
What do they look like?
Why are/ aren’t they useful?
Shows the covalent bonds as single lines between atoms
- shows a single bond, = shows a double bond and so on
Useful to show how atoms are connected in large molecules
Doesn't show the 3D structure of the molecule
Doesn’t show which atoms the electrons in the covalent bond have come from
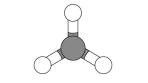
3D model for covalent bonds:
What do they look like?
Why are/ aren’t they useful?
Shows the atoms, the covalent bonds and their arrangement in space next to each other
Can get confusing for large molecules where there are lots of atoms to include
Don't show where the electrons in the bonds have come from
How do you find the empirical formula of the covalently bonded compound from these diagrams?
Count the number of each element
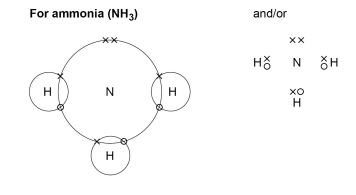
How is ammonia different to ammonium?
Ammonia - NH3
the nitrogen needs to gain 3 electrons and the hydrogen only 1
they form 3 single covalent bonds
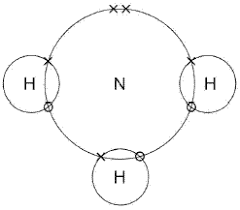
Ammonium - NH4
same thing happens, but a 4th hydrogen loses its only electron to the surroundings
it needs 2 more electrons for a full outer shell
it shares nitrogen’s final two electrons to do this
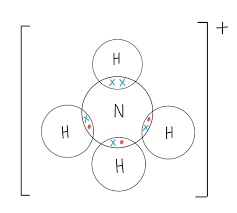
What are the properties of simple molecular substances?
low MPs and BPs
mostly gases at room temp
don’t conduct electricity
bigger simple molecular substances have higher MP and BPs
What are IMFs?
Intermolecular forces
What are the electrostatic forces?
Attraction + repulsion
Why do simple molecular substances have low MP and BPs?
There are weak IMFs between the molecules
Why do larger simple molecular structures have higher MPs and BPs?
The IMFs increase with the size of the molecules
Why don’t simple molecular substances conduct electricity?
The molecules do not have an overall electric charge
Give an example of a covalently bonded substance with large molecules
Polymers
What are polymers?
Long chains of repeating units
How are polymers formed?
Lots of small units are linked together to form a long molecule that has repeating sections
The atoms are linked to other atoms by strong covalent bonds
How do you draw the displayed formula of a polymer?
N is the number of times the section repeats
the repeating unit is in the brackets
the bonds on the outside are both in and out of the brackets
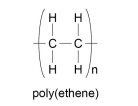
What are the properties of polymers?
Strong IMFs, so have high MPs and BPs
Solid at room temp
IMFs are still weaker than ionic or covalent bonds, so have lower MPs and BPs than ionic or giant molecular compounds
What are macromolecules?
Giant covalent molecules
What are the properties of giant covalent structures?
High MPs and BPs
Solids
Don’t conduct electricity (even when molten) due to no charged particles
Why do giant covalent structures have high MP and BPs?
no IMFs, only covalent bonds
these require lots of energy to break
Give 3 examples of covalently bonded substances that have giant covalent structures
diamond
graphite
silicon dioxide (silica - what sand is made of)
What do metals consist of?
Giant structures of atoms arranged in a regular pattern
How are metallic bonds formed?
The electrons in the outer shell of metal atoms are delocalised and so are free to move through the whole structure
This creates positively ionic metals
The electrostatic forces of attraction are between the positive metal ions and delocalised electrons
Diagram of metallic bonding
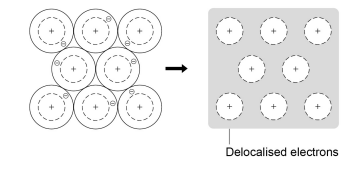
What are the MP and BPs of metals like?
Mostly high due to strong metallic bonds
How are atoms arranged in pure metals?
In layers
What do the layers of atoms in metals allow for?
Can be bent and shaped as the layers can slide over each other
Why are alloys used more often than pure metals?
Pure metals are too soft for many uses
Mixing them with other metals to create alloys makes them harder
Why are alloys harder than pure metals?
The atoms are different sizes, so the layers are distorted
this means the layers can slide over each other less easily
Why are metals good conductors of electricity?
The delocalised electrons in the metal carry electrical charge through the metal
Why are metals good conductors of thermal energy?
Energy is transferred by the delocalised electrons
What are the three states of matter?
solid
liquid
gas
What takes place at melting point?
melting and freezing
What takes place a boiling point?
boiling and condensing
Particle model of the three states of matter

What does the amount of energy needed to change state from solid to liquid and liquid to gas depend on?
The strength of the forces between the particles of the substance
What does the nature of the particles involved depend on?
The type of bonding and the structure of the substance
How do the strength of the forces between particles affect the MP and BP?
The stronger the forces between the particles the higher the MP and BP
What are the limitations of the solid sphere particle model?
There are no forces
Particles are represented as spheres
The spheres are solid and inelastic
What are the state symbols for chemical equations?
solid (s)
liquid (l)
gas (g)
aqueous (aq)
Give 4 allotropes of carbon
diamond
graphite
graphene
the fullerenes
Describe the structure of diamond
each carbon forms 4 covalent bonds with other carbon atoms
giant covalent structure
What are the properties of diamond?
Hard
Very high MP
Doesn’t conduct electricity
Describe the structure of graphite
Each carbon atom forms 3 covalent bonds with 3 other carbon atoms
Forms layers of hexagonal rings
No covalent bonds between layers
Why is graphite conductive?
One electron from each carbon atom is delocalised
Why is graphite soft and slippery?
The layers can slide over each other
What is graphene?
A single layer of graphite
What does graphene’s properties make it useful in?
Electronics and composites
Why is graphene so strong?
The atoms in the layers are tightly bonded covalently
Why is graphene elastic?
The planes of atoms can flex relatively easily without the atoms breaking apart
What are fullerenes?
Molecules of carbon atoms with hollow shapes
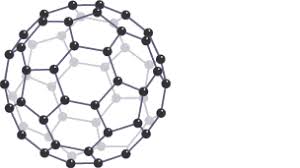
What is the structure of fullerenes based on?
Hexagonal rings of carbon atoms
(may also contain rings with five or seven carbon atoms)
What was the first fullerene to be discovered?
Buckminsterfullerene (C60)
What shape does buckminsterfullerene have?
Spherical
What are carbon nanotubes?
Cylindrical fullerenes with very high length to diameter ratios
What do the properties of carbon nanotubes make them useful for?
Nanotechnology
Electronics
Materials
What can the fullerenes and carbon nanotubes be used for?
Lubricants
To deliver drugs in the body
Catalysts
What can nanotubes specifically be used for?
Reinforcing materials (e.g. tennis rackets)
What does nanoscience refer to?
Structures that are 1-100 nm in size
How many atoms do nanoparticles contain?
A few hundred
Are nanoparticles or fine particles smaller?
Nanoparticles
What is the short form for fine particles?
PM2.5
What diameters so fine particles have?
Between 100 and 2,500 nm