Unit 2 Class 1 - Catecholamines pt 1
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
issues with correlating neurological disorders with NTs
the reliance on classifying neurological disorders based on NT transmission solely leads to a limited perspective on the disorder
one to one links between diseases and NTs do not exist, it is an antique of the pharmacology industry trying to sell drugs
ex: serotonin being linked to depression
catecholamines
amines
dopamine (DA)
norepinephrine (NE)
epinephrine (EPI)
dopamine synthesis
tyrosine -tyrosine hydroxylase → dopa -dopa decarboxylase → dopamine
norepinephrine synthesis
dopamine -DBH→ NE
rate limiting enzyme in dopamine and NE pathway
tyrosine hydroxylase
tyrosine hydroxylase regulation
high catecholamine levels in the terminal inhibits tyrosine hydroxylase (negative feedback)
neuron firing rate of action potentials: more firing will lead to more TH synthesis
second messenger associated proteins (PKA, PKG, PKC, CaMK) stimulates TH
how to activate and inhibit catecholamine synethesis through drugs
enhancing: administering precursors such as tyrosine or L DOPA (to treat Parkinson’s disease since dopamine in its final form cannot cross the blood brain barrier)
→ patients treated w L DOPA should be treated with carbidopa (blocks dopamine carboxylase) so dopamine is not synthesized outside of BBB)
inhibiting: AMPT blocks TH which blocks synthesis of catecholamines
dopamine deficient mouse significance
dopamine deficient mouse genetically knocked out the TH gene which led to inability to synthesize dopamine (but restored it in DBH expressing genes so the mouse could make NE)
significance: led to lack of eating, drinking, hypoactivity, and underweight, but could be restored with L DOPA administration
→ Dopamine = developmental success
vesicular monoamine transporter (VMAT)
carries catecholamines into vesicles (enzymes will break them down otherwise)
> VMAT 1 = adrenal medulla/kidney
> VMAT 2 = brain
VMATs can be blocked by the drug reserpine (old antipsychotic) to keep catecholamines out of vesicles
mechanism and effect of reserpine
if NE and DA is not protected in the cell they will be broken down by enzymes
This will lead to depressive like behavior that can be reversed with DOPA (DA precursor)
significance: catecholamine theory of depression
amphetamine mechanism
cellular effect: causes catecholamine release independent of cell firing
behavioral effect: increased locomotor activity and steryotyped behaviors (sniffing, head twitching) s a direct result of increased DA
how is catecholamine release inhibited
autoreceptors on cell bodies, dendrites, and terminals
→ for DA specifically, autoreceptors are G coupled protein receptors that lead to K+ channels opening (hyperpolarizes the cell and leads to shorter AP and inhibits VGCC to prevent excytosis)
autoreceptor subtypes for DA and NE
DA autoreceptor = D2
NE autoreceptor = a2
ex: mutant mice w no D2 receptors were more active and sensitive to cocaine than controls
drug interactions at catecholamine autoreceptors
autoreceptor stimulus = catecholamine inhibition
autoreceptor antagonists = enhanced release of catecholamine
clonadine (catapres)
anxiety caused by opiate withdrawal stimulates noradernergic pathway (anti reward pathway)
clonadine enhances a2 autoreceptors to inhibit NE
yohimibine
a2 antagonist that increases noradernergic cell firing and NE release
reuptake (catecholamines)
DA + NE move from synaptic cleft into nerve terminal via specific membrane transporter proteins DAT and NET for repackaging/breakdown
ex: mutant mice with no DAT do not respond to psychostimulants like cocaine
degredation of catecholamines (know the types of enzymes and metabolites)
occurs through enzymes MAO and COMT
MAO-a: metabolizes NE
MAO-b: metabolizes DA
COMT: metabolizes NE and DA
DA metabolite: HVA
NE metabolite: MHPG (brain) or VMA (PNS)
→ these metabolites enter CSF and bloodstream and are excreted in urine
metabolic pathway
be able to draw this out for both DA and NE
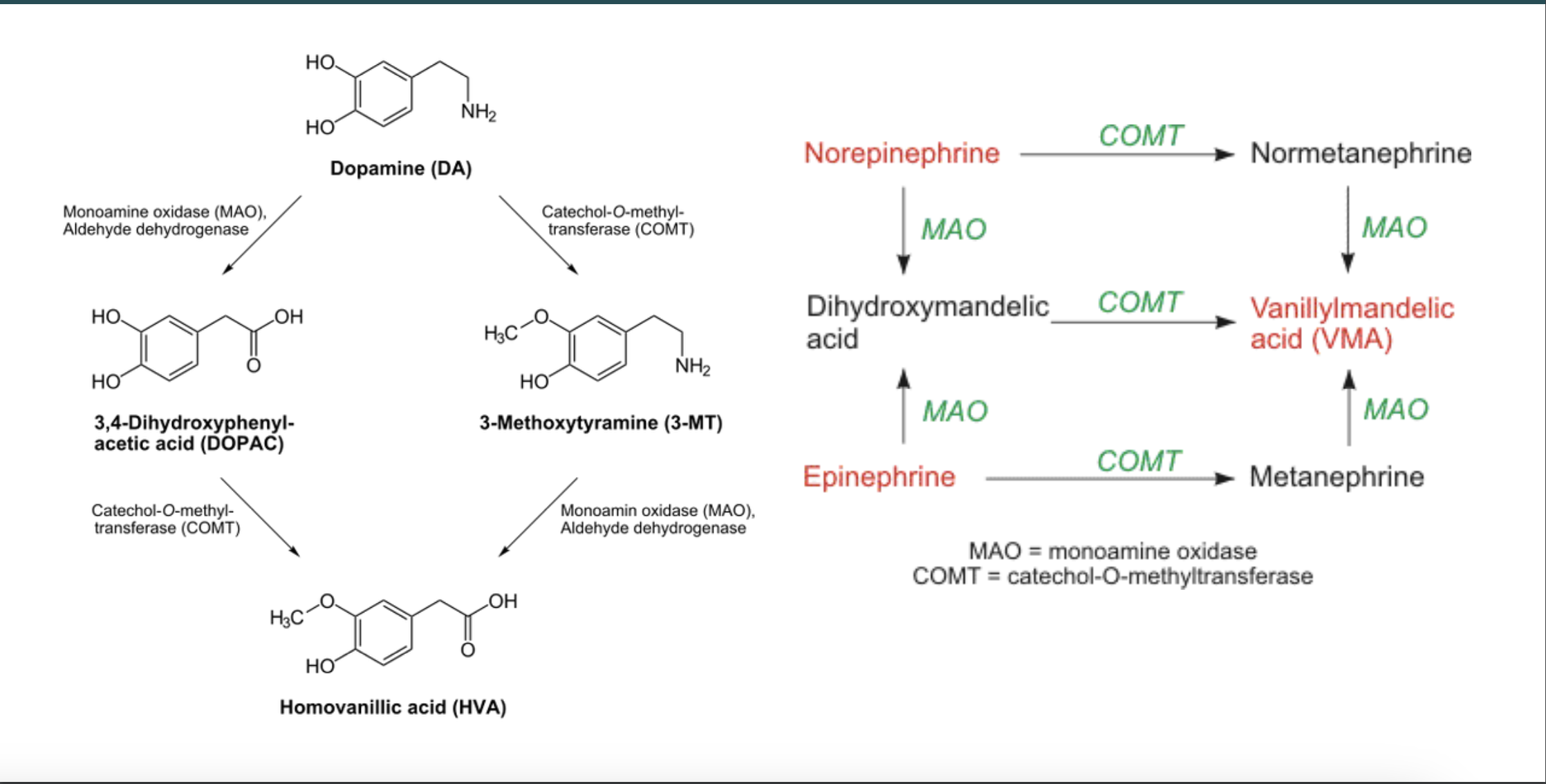
which drugs inhibit the enzymes that metabolize catecholamines
MAO inhibitors like phenelzine (Nardil) and tranycypromine (Parnate) are used to enhance catecholamine levels (antidepressants)
COMT inhibitors like entacapone (Comtan) and tolcapone (Tasmar) enhance the effectiveness of L DOPA in treating Parkinson’s disease
where do the three ascending dopaminergic system pathways originate
brainstem
dopamine nigrostriatal tract
substantia nigra → striatum
these cells die along the progression in Parkinson’s Disease (leading to cell death in the striatum)
dopamine meso tracts (2)
mesocrotical dopamine pathway: VTA → prefrontal cortex and hippocampus
affects cognition, working memory, attention, implicated in schizophrenia and ADHD
mesolimbic pathway: VTA → NAc (and various structures in the limbic system)
reward processing, arousal, locomotion, implicated in addiction
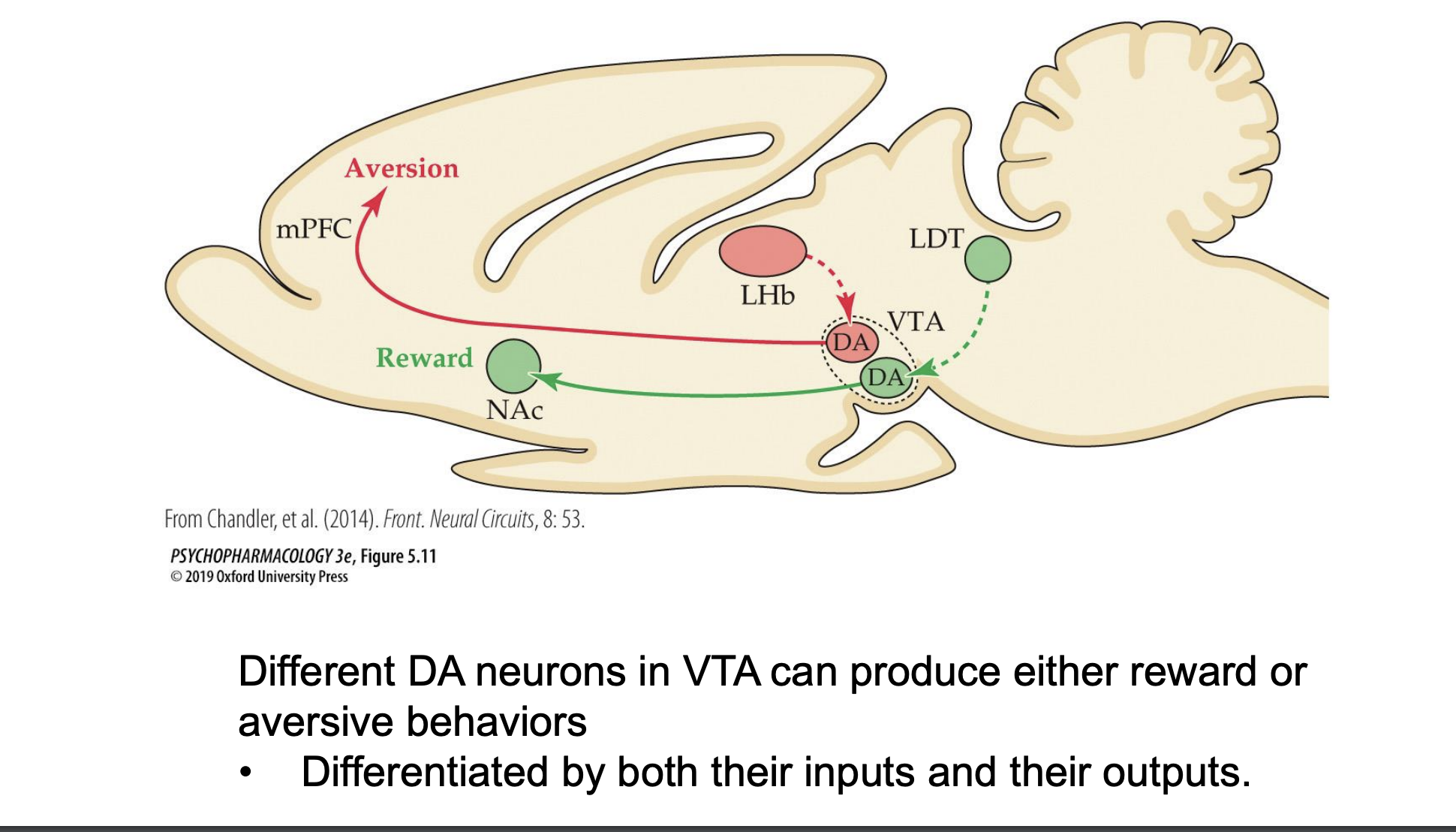
different effects of DA neurons in the VTA
different DA neurons in the VTA can produce both reward and aversive behaviors differentiated by both their inputs and outputs
different DA receptor subtypes and where they are found
five in total D1, D2… D5, all metabatropic
> split into D1 like and D2 like
D1 like is D1 and D5: Gs or Gq protein (excitatory, stimulates adenylyl cyclase + cAMP production)
D2 like is D2, D3, D4: Gi/o protein (inhibitory, usually autoreceptors to inhibit adenylyl cyclase and cAMP by regulating calcium/potassium channels)
primarily found in NAc and dorsal striatum
D1-D2 heteromers
a complex of both types of dopamine receptors with its own Gq protein and two binding sites
agonists and antagonists can selectively target these receptors
DA affinity to subtypes (tonic and phasic bursts)
dopamine has higher affinity to D2 receptors than D1 receptors (10-100x higher)
phasic (burst): DA firing engages D1 receptors (in addition to D2)
tonic (slower): DA firing engages only D2 receptors
structures involved in basal ganglia
striatum
globus pallidus (GPexternal/GPinternal)
subthalamic nucleus
substantia nigra (split into SNR and SNC)
direct pathway of movement
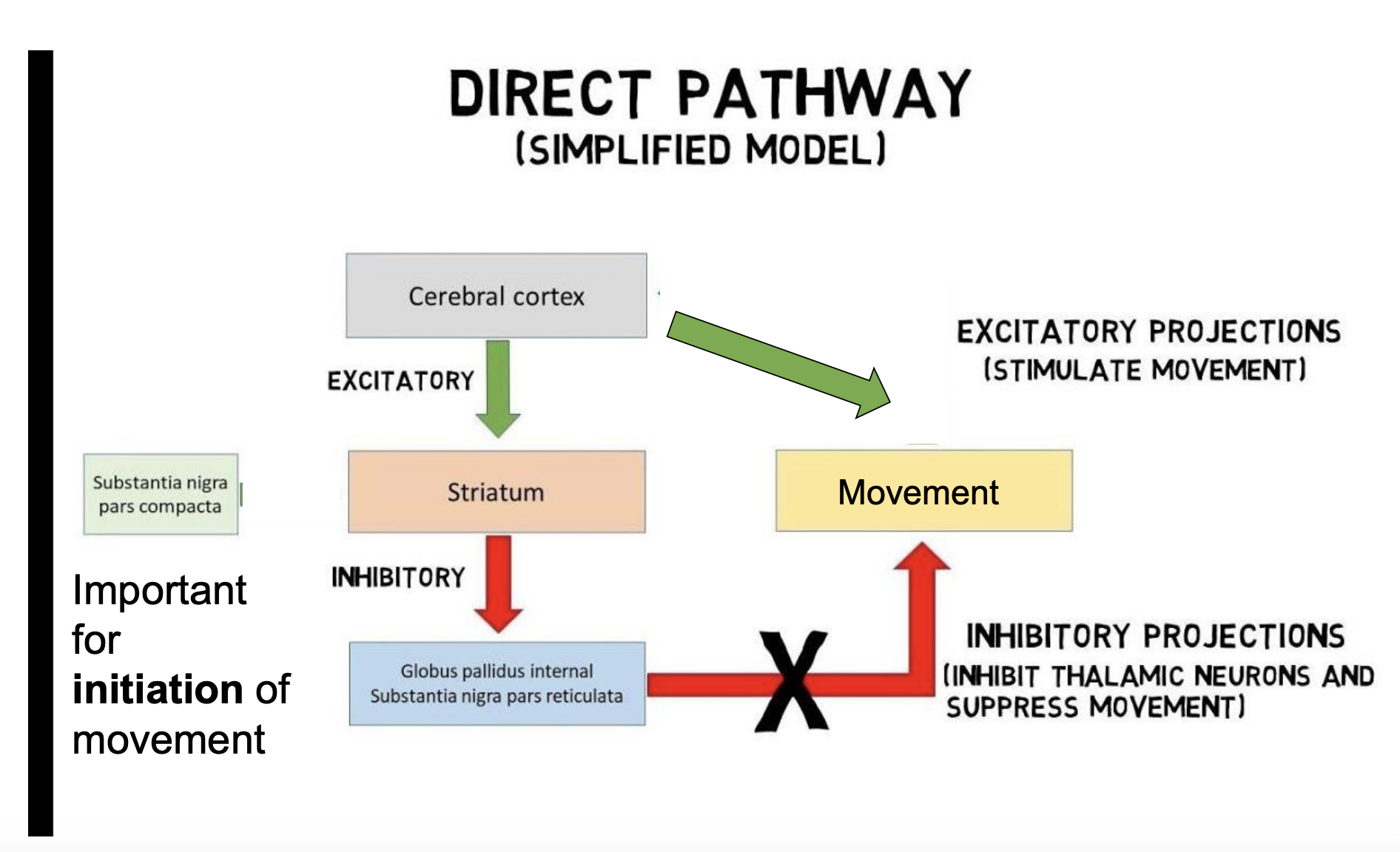
important for initiation of movement, be able to draw
indirect pathway of movement
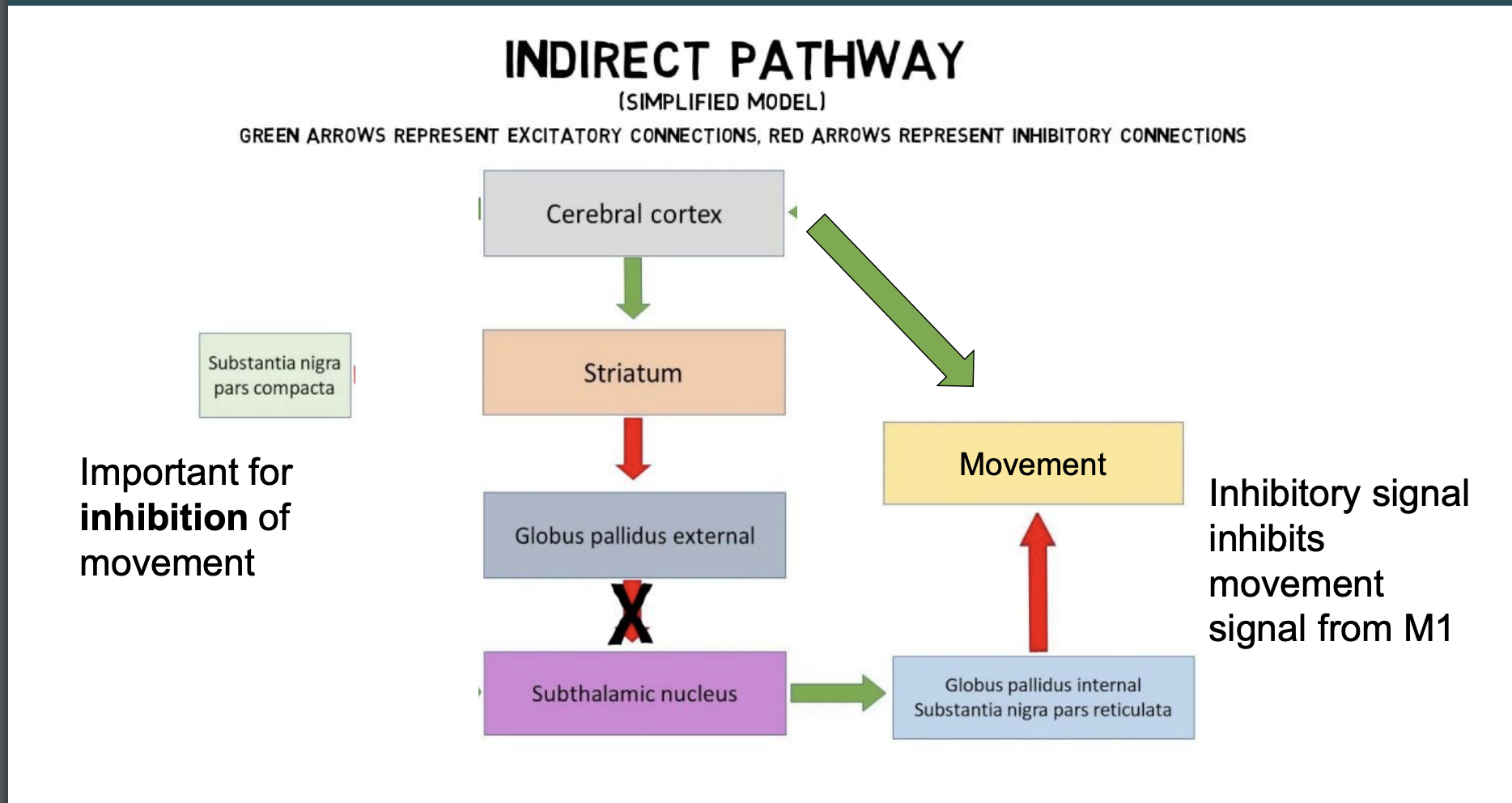
important for the inhibition of movement, be able to draw
why does dopamine regulate the movement pathways
both the direct and indirect pathways can send competitive signals, and the indirect pathway will usually win
→ dopamine guides both pathways in different ways but the effect is the same (movement is enhanced)
central noradernergic system
cell bodies in the brainstem (pons and medulla) → forebrain
implicated in arousal, cognition, consolidation of emotional memories
maintaining/transferring to wakefulness caused by NE neurons stimulated by orexin neurons
peripheral noradernergic system
sympathetic nervous system that causes fight or flight reaction
locus coeruleus
NE neurons made in pons and medulla
locus coeruleus is a dense collection of NE neurons in the pons that extend to nearly all areas of cerebellum, forebrain, spinal cord
adrenoreceptors
metabatropic, subtypes a and B
a1 receptors: Gq, enhances cAMP production through PLC3, IP3, and DAG
a2 receptors: Gi/o, inhibits cAMP production (autoreceptors)
B1 and B2: Gs protein, enhances cAMP production through stimulating adenylyl cyclase
NE and working memory
LC → PFC projections disrupts attention and working memory
a2 agonists like guanfacine are a promising treatment for ADHD
what levels of NE optimizes working memory
intermediate
stress (lots of NE) or sedation (no NE) = less cognitive functioning
what does NE have higher affinity for
a2 than a1