Chapter 17 Reactions of Aromatic Compounds
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
37 Terms
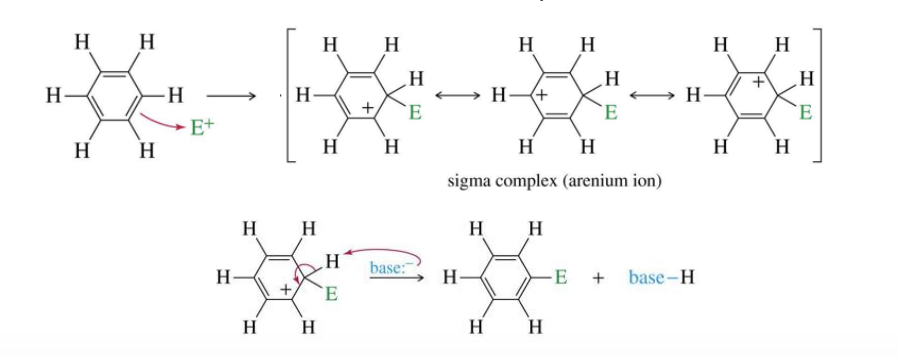
Electrophilic Aromatic Substitution (EAS)
When an aromatic ring reacts with a base with a negative charge to gain an electrophile (stays aromatic)
loses aromaticity during the intermediate cation, but the product is aromatic
carbocation intermediate/sigma complex is resonance stabilized
can be any aromatic ring, not only benzene
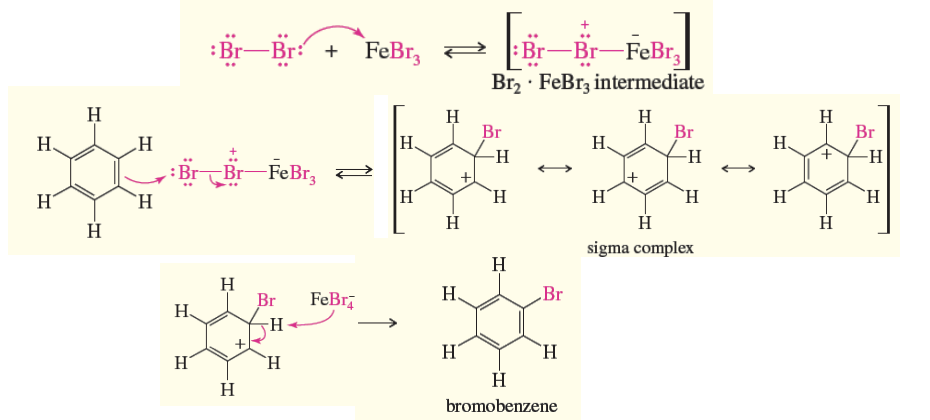
Benzene Bromination (EAS)
Benzene + FeBr3 —> carbocation/sigma complex —> deprotonated by FeBr4 to gain bromobenzene
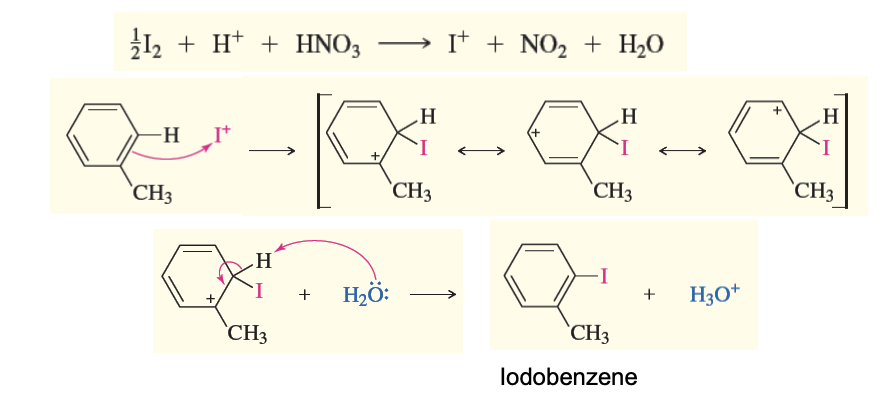
Iodination of Toluene or Benzene (EAS)
Iodine (I) + Nitric acid (HNO3) react with toluene replacing H with I
to start the reaction iodine must react with HNO3 to form I+ that reacts with the benzene
carbocation intermediates / sigma complexes that are resoancne stabalized
deprotonation by h20
EAS
creates iodobenzene
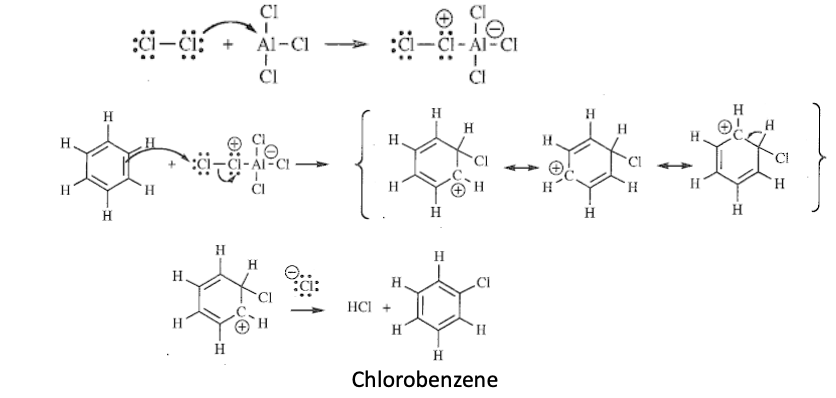
Benzene Chlorination (EAS)
Aluminum chloride (AlCl3) + Cl2 —> creates AlCl5 reagent
Benzene + AlCl5 reagent —> sigma complexes with carbocation —> deprotonated by Cl- —> chlorobenzene formed
Activating groups
They are electron donating groups that help direct the substitution mechanism
Ortho and Para directing substitutents that are activating groups
Alkyl (R), Alkoxy (RO), and Amine Groups (NH2)
R: push induction for stable carbocation
RO: does resonance stabilization of sigma complex
NH2: does resonance stabilization of sigma complex
(NOTE THAT R IS JUST CARBON CHAINS EX METHANE, EHTANE, ETC.)
They all direct in favor ortho and para positions that have stable tertiary carbocation
Order of ortho and para directing substituents
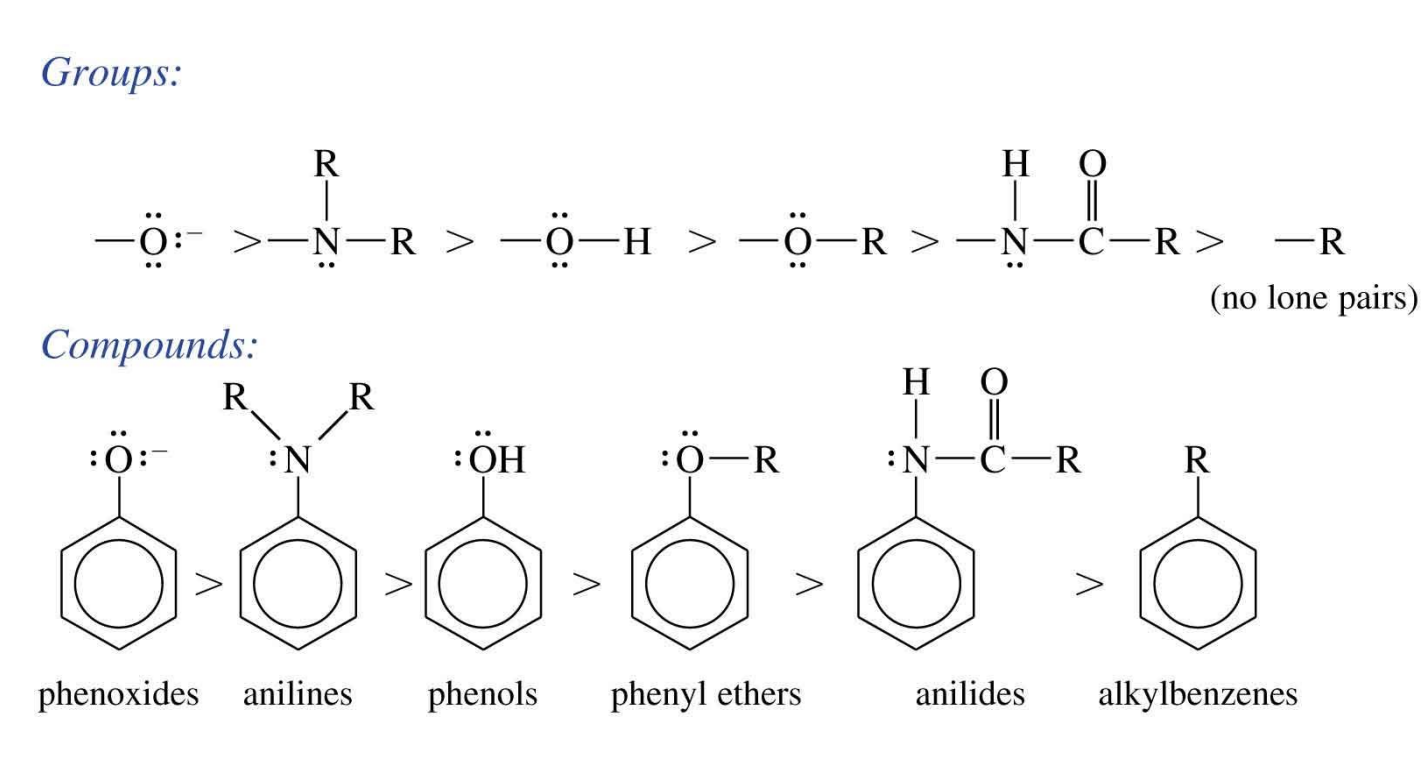
Remember the groups: O > NR2 > OH > OR > NHCOR > R
Deactivating groups
They are electron-withdrawing groups substituents that push for the meta position product
Meta-Directing Substituents
The deactivating groups (electron withdrawing) push for meta attacks
MOST COMMONLY USED IS NO2
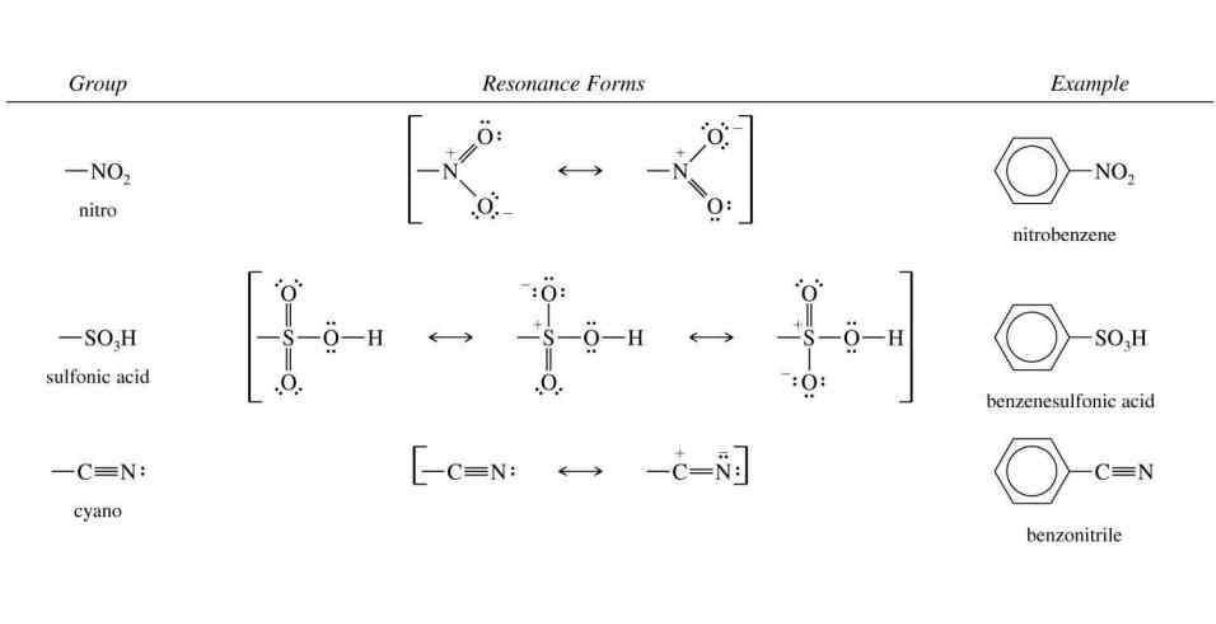
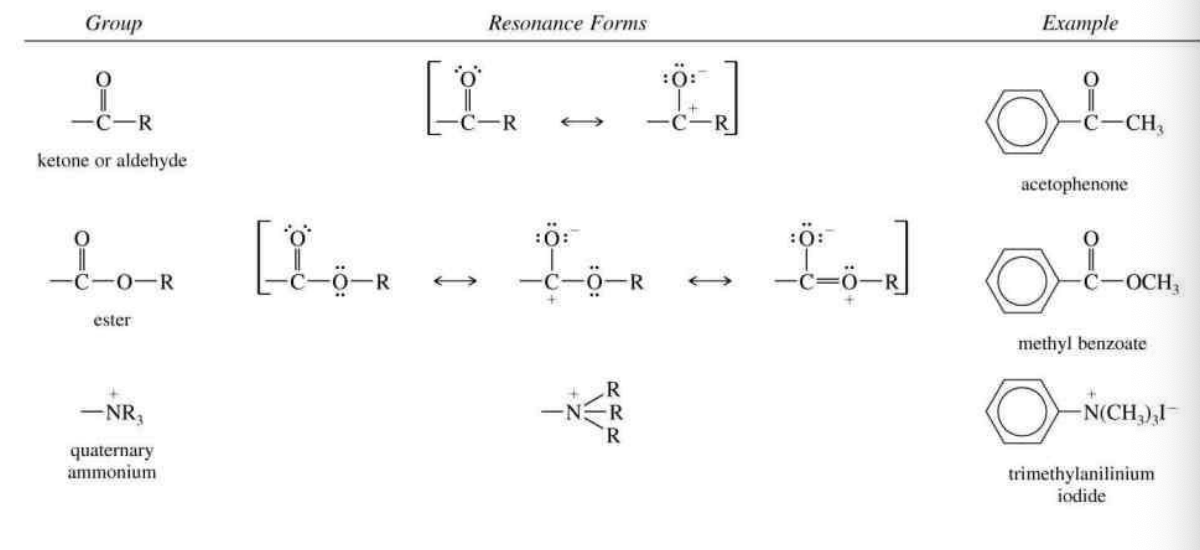
Seperate by carbonyl groups: COOH, COOR, COR, CRR
And others: NO2, CN, SO3H, N+R3
Halobenzenes
Refers to benzene attached to any halogen (Br, F, I, Cl)
They are electron-withdrawing groups (meta?) but halogens have lone pairs (ortho/para?)
lone pairs resonance stabalize sigma complex
halogens electron withdrawing deactivates the benzene (less reactive)
their net effect cancels out so they are ORTHO AND PARA DIRECTING
Ortho and para: resonance stabalized by charged
Meta: pos charge placed on halogen’s carbon —> unstable
Multiple Substituents (ortho and para activators vs meta activators)
Ortho & para activators take priority over meta activators
Strong ortho & para activators: NH2> OH > OR
Moderate ortho & para activators: R (alkyl) > Halogens
Meta Directors: NO2, CN, Ket & alde, etc.
meta directors have the smallest effect
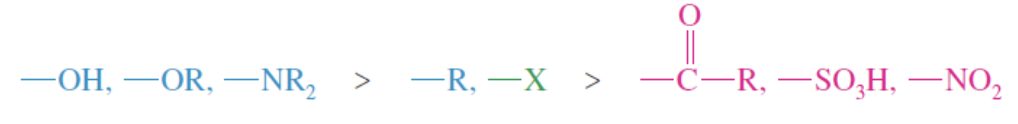
Multiple Substituents of the same type of activtor and strength
If a benzene has 2 substituents that are both activating or both deactivating and they want the new group to be attached to different reaction sites —> it produces mixed products
same class activator or deactivator on benzene —> mixed products
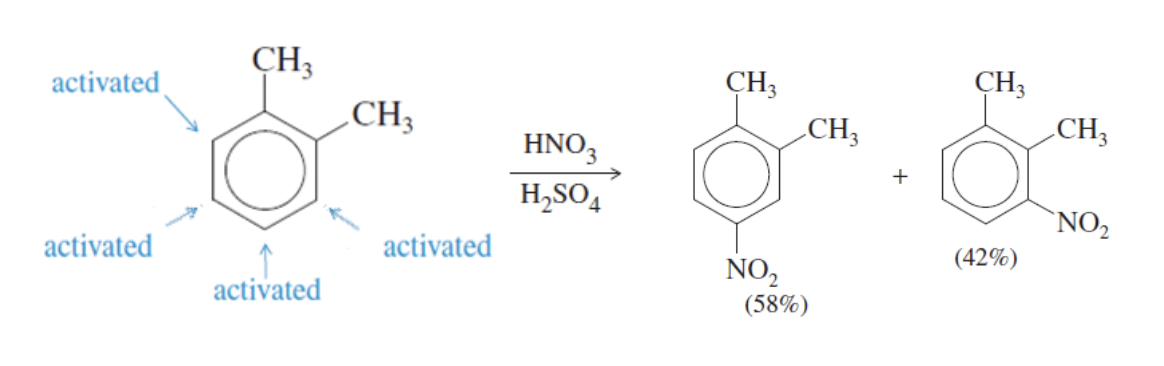
Multiple Substituents Reinforcing vs Opposing
Reinforcing positions → product is obvious
if the original structure has substituents that push new group to the same type of reaction site (same products)
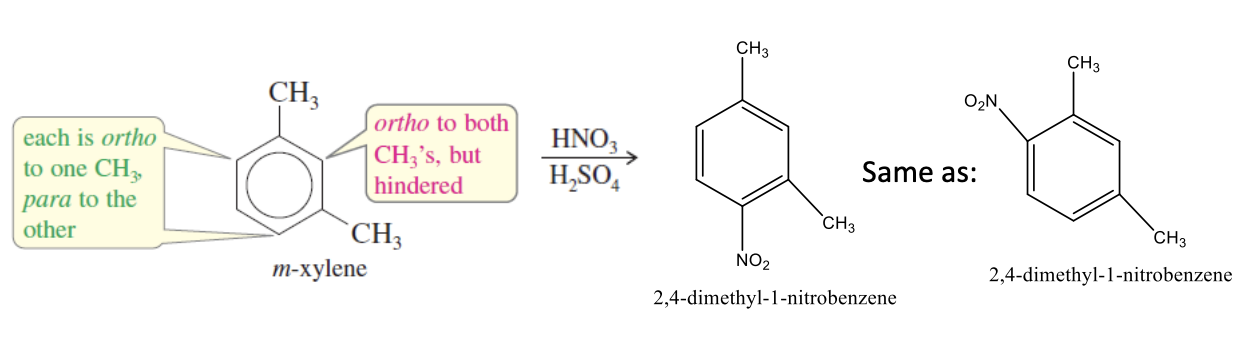
Opposing → strong activator wins
if the original structure substituents direct a new group to different type of reaction sites (the product enforced by the storngest activator wins)
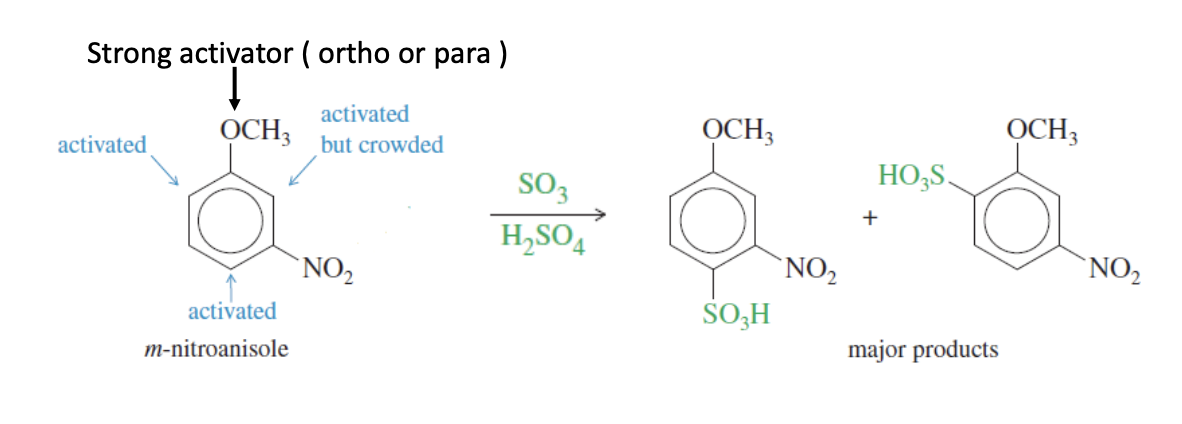
Friedel-Crafts Alkylation
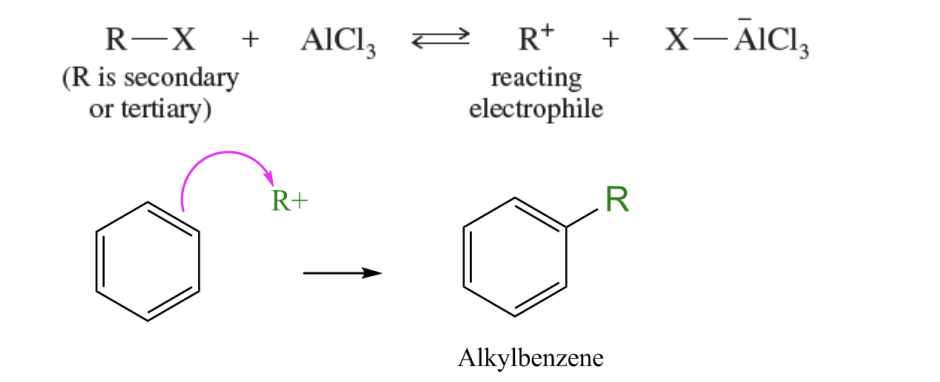
Sec or Tert alkyl halide + AlCl3 or FeCl3 —> alkyl carbocation —> Benxene + alkyl carbocation —> sigma complex resonance stabalized —> alkylbenzene
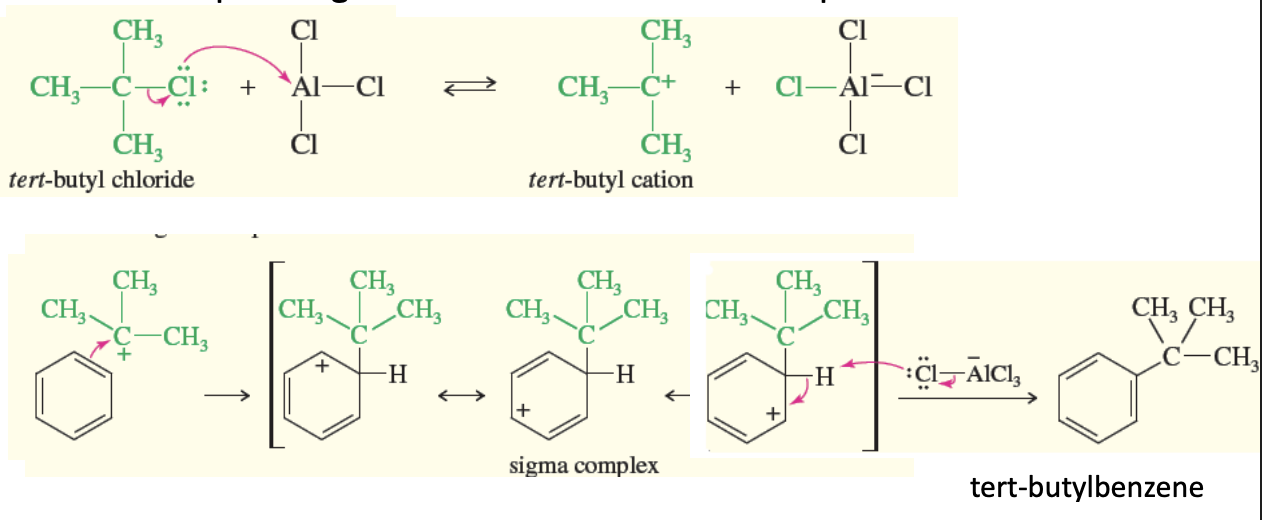
Friedal-Crafts Alkylation Carbocation Sources
Carbocation can form from 4 things
R-X + AlCl3 —> R+ + x-AlCl3
R-X + FeCl3 —> R+ + x-FeCl3
Alkene + HF —> Protonated Alkene + F-
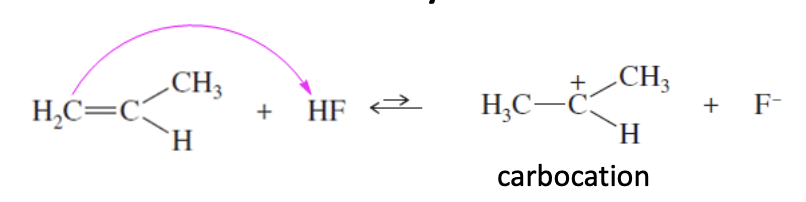
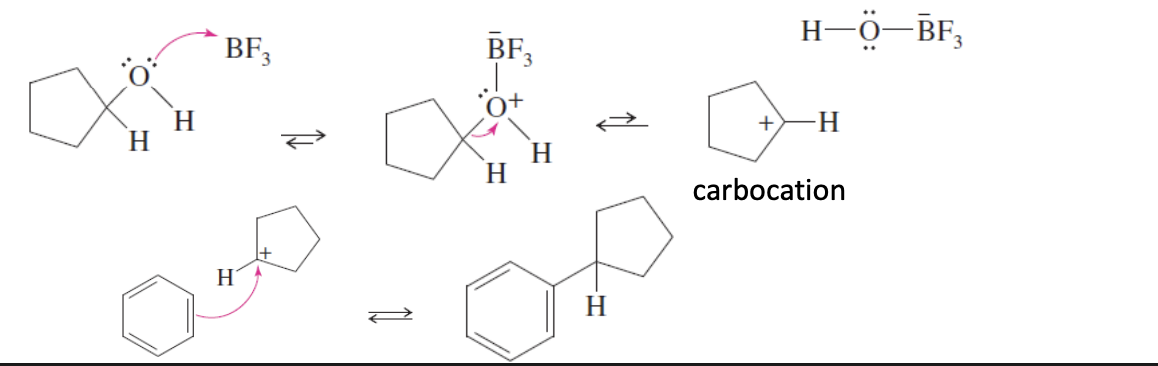
Alcohol + BF3 —> Carbocation +OHBF3
Friedel-Crafts Alkylation Limitations
Only works on reactive rings (benzene, benzenes with electron donating groups, & halobenzenes)
CANNOT WORK WITH STRONGLY DEACTIVATING SUBSTITUENTS (benzens with electron withdrawing groups)
Carbocation rearrangements are possible —> more stable carbocation that changes the final product
The alkyl groups can activate the ring, resulting in multiple alkylations
Reactive ring dependent, carbocation rearrangements, multiple alkylations
Friedel Crafts Acylation
Acylbenzene is formed
carboxycylic acid + socl2 —> +alcl3 → r-c=o +alcl4
r-c=o + benzene ring —> sigma complex —> +alcl4 —> acylebenzene
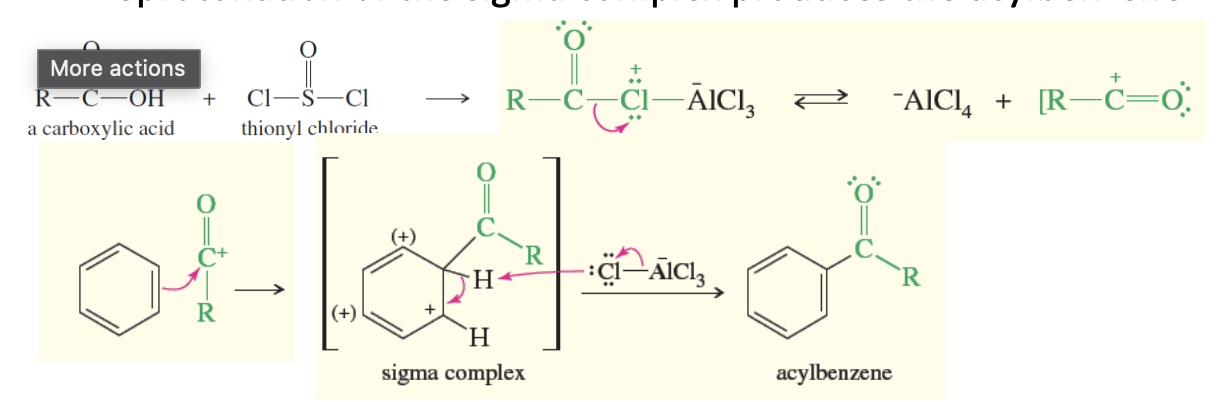
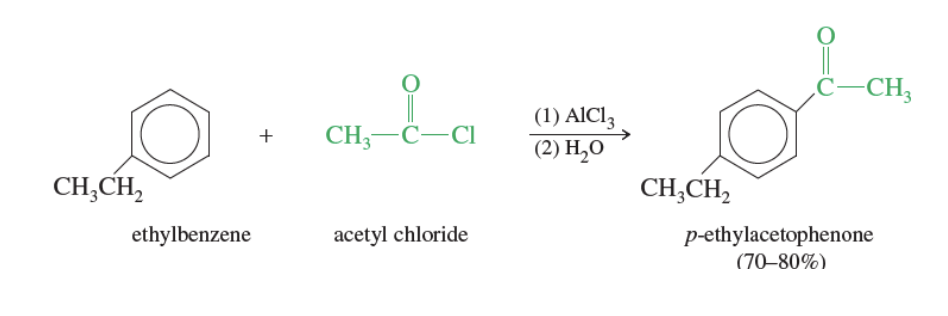
Rules for Friedel-Crafts acylation
Only reacts with benzene, benzenes with electron donating groups, and halobenzenes
CANNOT REACT WITH STRONG DEACTIVE SUBSTITUENTS (electron-withdrawing groups or meta position drivers)
Favors Para substitutions
ortho possible but it has steric hinderence
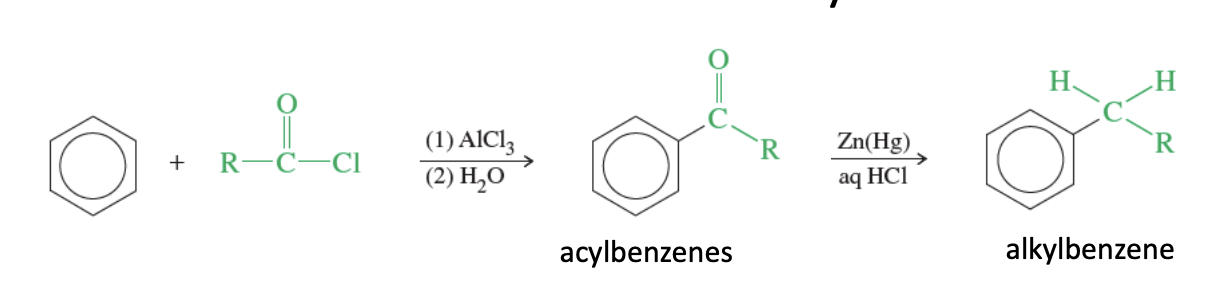
Clemmensen Reduction
Reducing an acylbenzene to an alkylbenzene
reduced using zincmercury and HCl
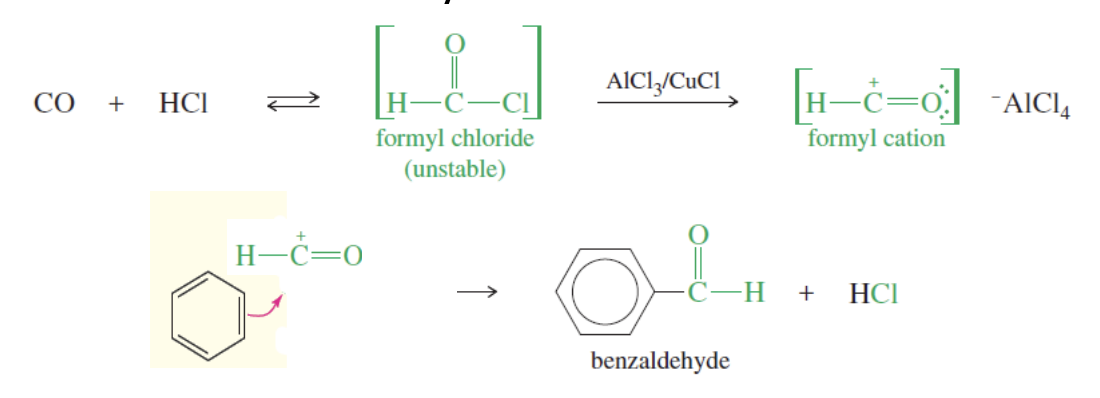
Gatterman-Koch Formylation
Like acylation but instead of using r-c=o it uses h-c=o
produces a benzaldehyde
Nucleophilic Aromatic Substitution
When a nucleophile replaces a benzene with 2 substitutents (strong electron withdrawing group and a leaving group
this leaving group tends to be a halogen
the nucleophile attacks the ring at the halogen site, preparing it for nucleophilic substitution
where nuc replaces halogen
in nucleophilic substitution, the EWG must be at the ORTHO OR PARA SITE
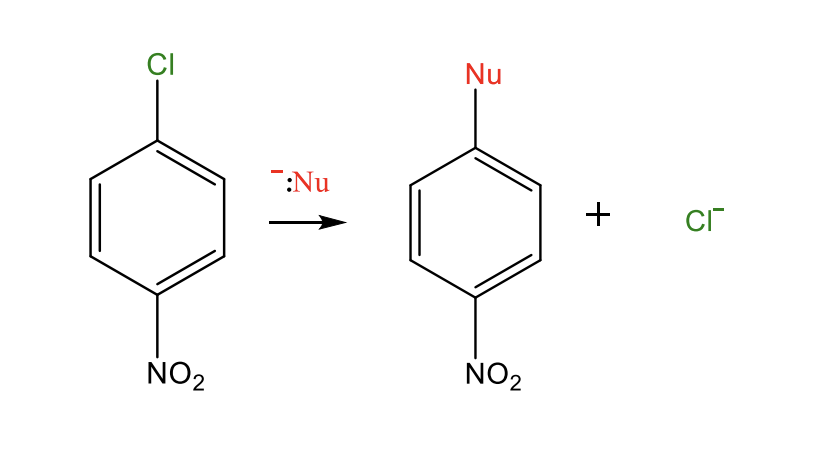
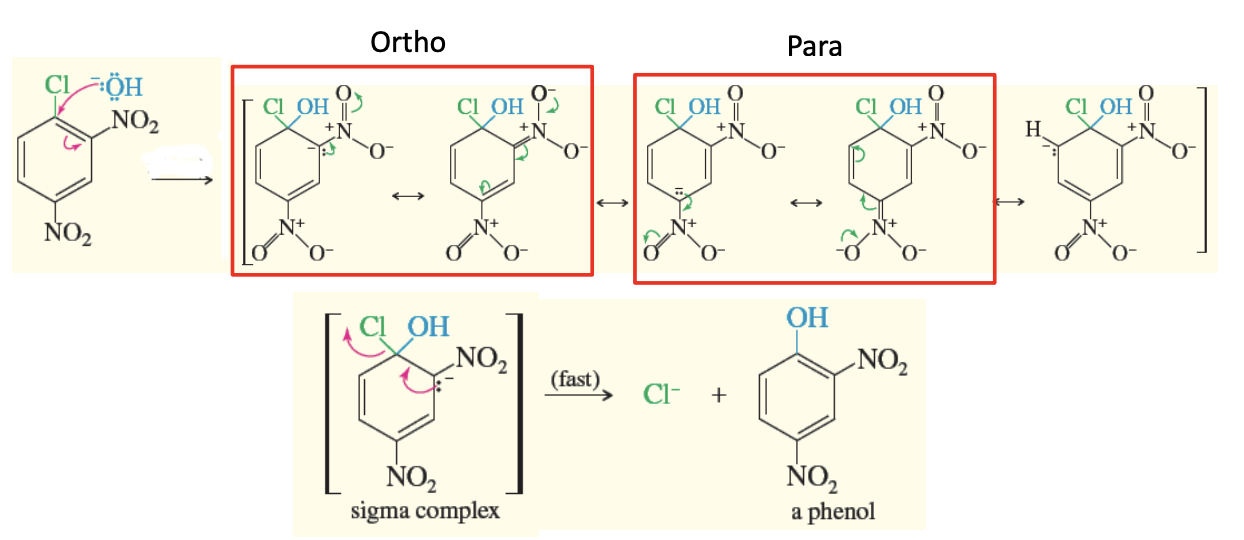
Addition-Elimination Mechanism
The nucleophilic aromatic substitution that occurs when the structure has 2 STRONG EWG (like 2 NO2’s attached)
the leaving group must still be either ortho or para (ortho to one ewg and para to the other ewg)
reacts with a nucleophile that will replace the leaving group halogen
it is a fast reaction
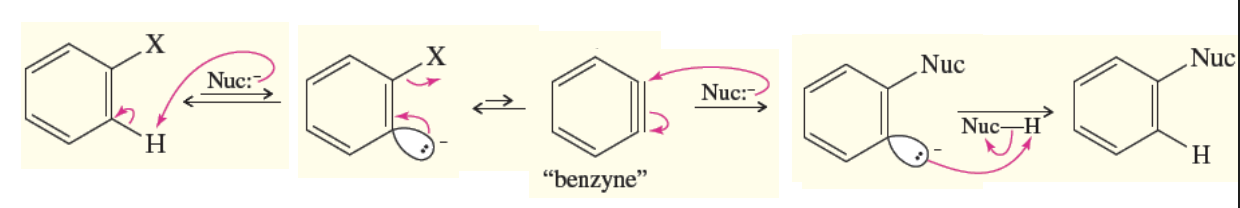
Elimination-Addition (benzene mechanism)
Occurs when a benzene has no strong EWG substituents and only a leaving group
results in an intermediate benzene and a final product of benzene with a nuc replacing the halogen (still nuc substitution)
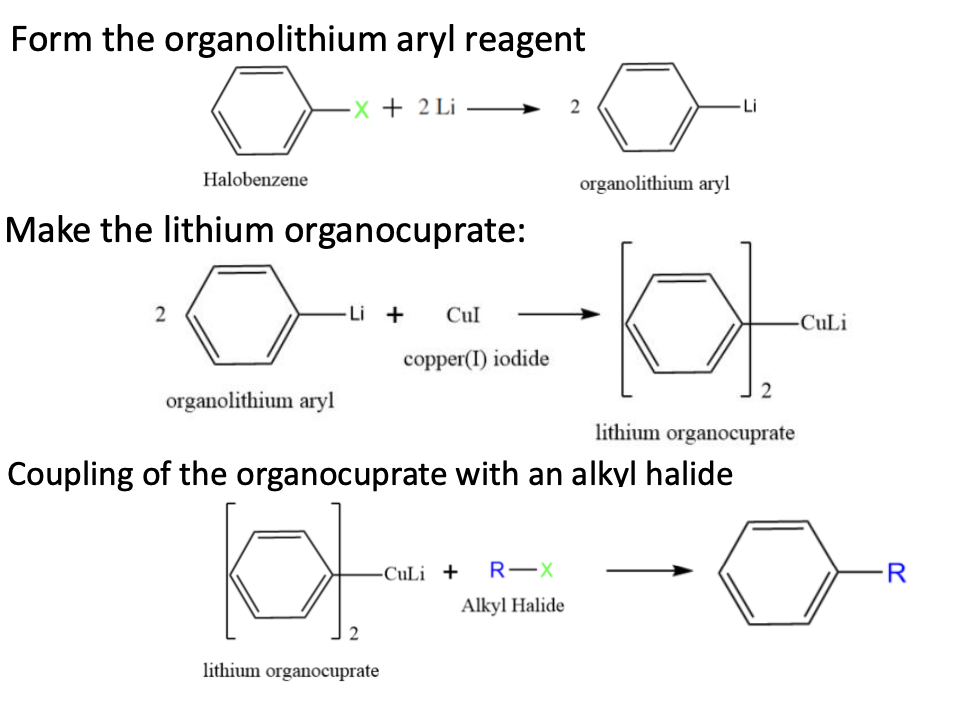
Organocuprate Reagents coupling
Requires aryl halide (formed by halobenzene + alkyl halide reacting together)
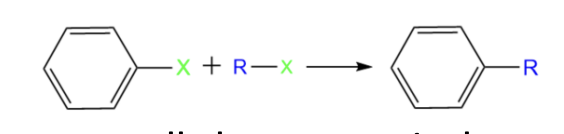
when an aromatic ring is coupled to an alkyl chain
First an Aryl Halide + 2 Li —> Aryl Lithium
Second 2 Aryl Lithium + CuI —> 2 ArylCuLi
This is the gilman reagent or the organocuprate (R2CuLi)
the reagent + Alkyl Haldie —> Aryl Alkyl final coupling product
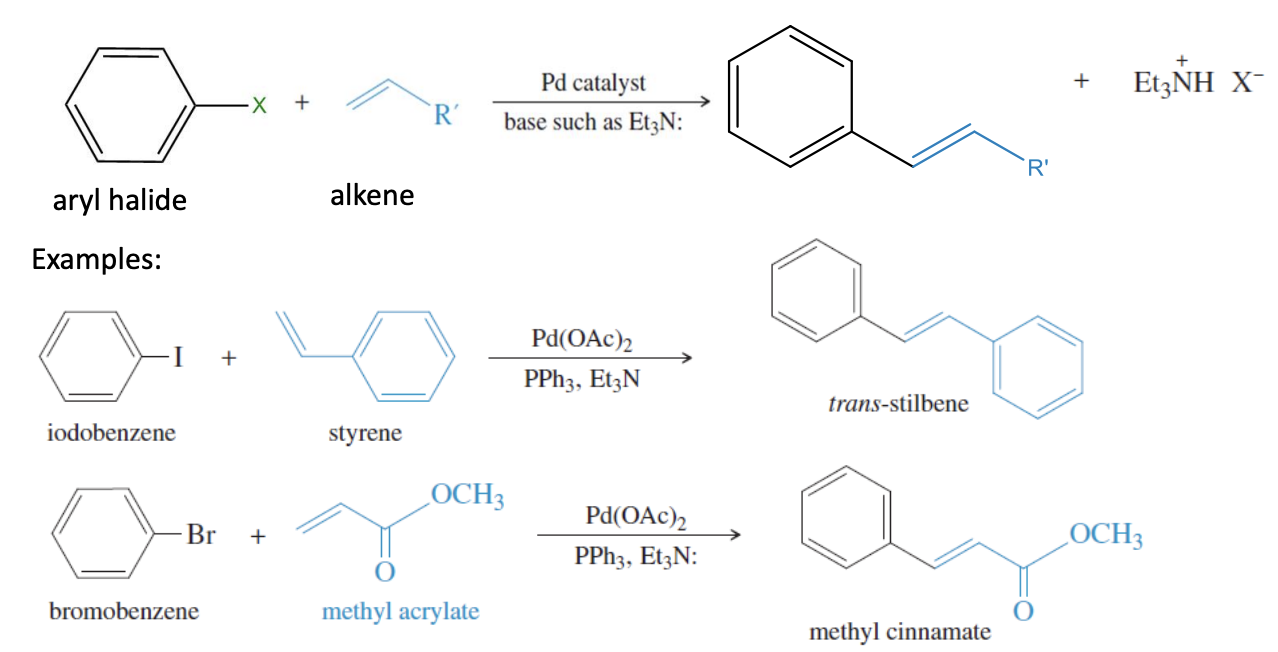
Heck Reaction
Aryl halide + alkene —> PdOAc2 catalyst with PPh3 Et3N —> coupled product
Addition Reactions
2 types: benzene chlorination and catalytic hydrogenation
Benzene Chlorination
When bezene treated with excess cl. (3Cl2)
done under heat and preasure or hv
producees bezne hexachloride (6 Cl’s attached)
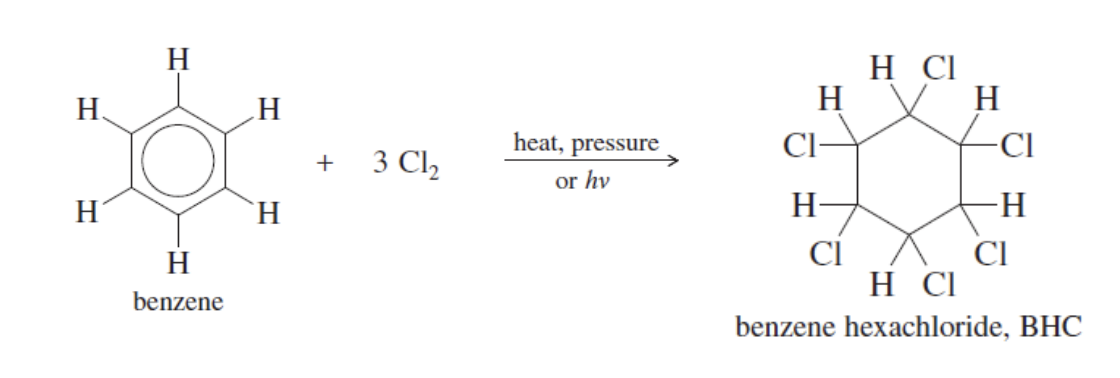
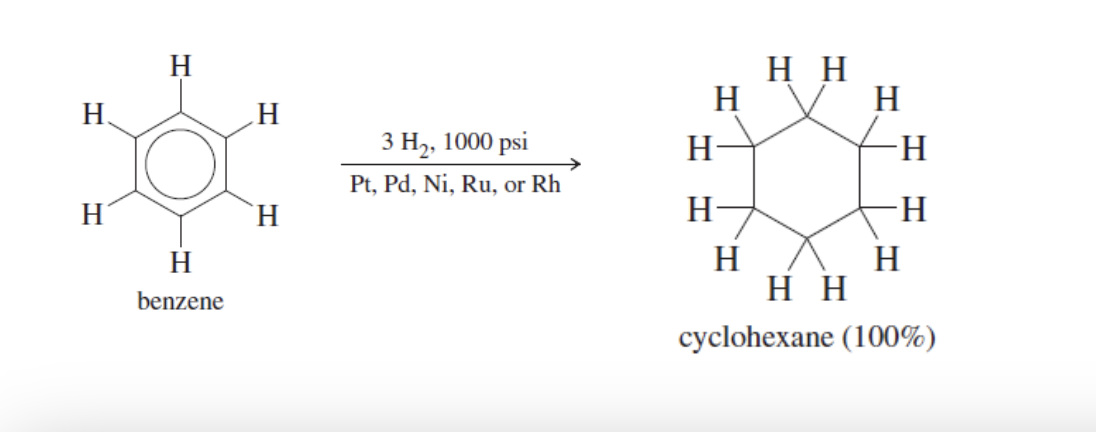
Catalytic Hydrogenation
Benzene reacts with excess hydrogen gas (3 H2)
done under heat and pressure + catalyst presence (Pt, Pd, Ni, Ru, Rh)
all carbons hydrogenated, produces cyclohexane
Side-Chain Reactions of Benzene
2 types: Permanganate Oxidation and Chlorination
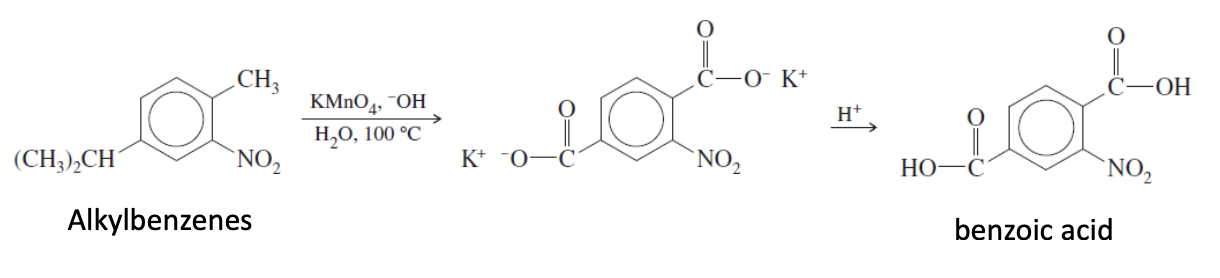
Premanganate Oxidation side chain benzene reaction
alkyl benzene + KMnO4, H20, H+ —> Benzoic acid
alkylbenzenes is when a benzens has 1 or more alkyl groups attached
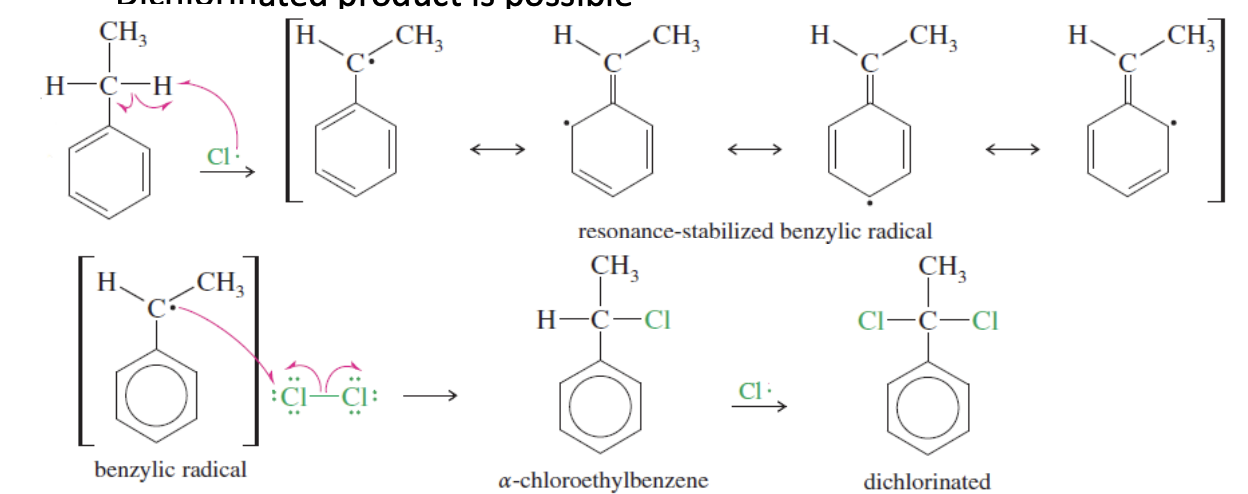
Chlorination Side chain free radical halogenation
Done with the goal of replacing the H’s on the benzylic carbon to Cl’s
Bromination
Ethylbenzene reacts with radical bromine —> resonance stabalized —> Br-Br —> Bromoethylbenzene
radical bromine formed with Br2 or NBS —> Hv —> Br radical
Nucleophilic Substition
Sn1 and Sn2 Reactions
Sn1 Reaction is carbocation nucleophilic substitution (2 step)
Sn2 Reaction is simple nucleophilic substitution
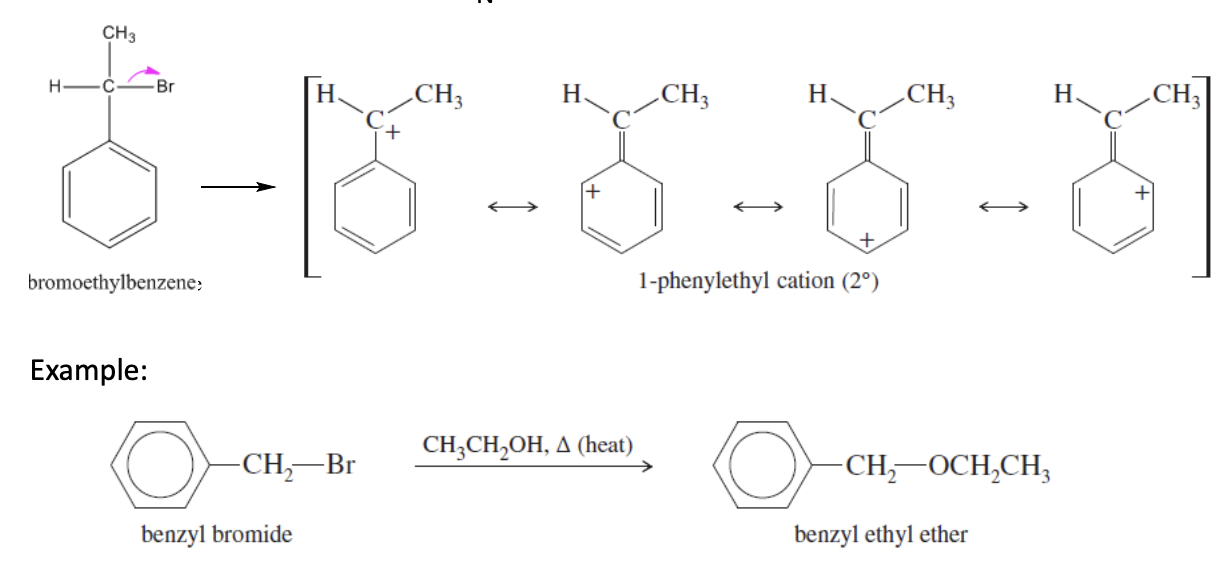
Sn1 Reaction with benzene
Benzene + nucleophile & heat —> Benzene carbocation intermediates —> Benzene + nucleophile
if the original structure is a benzyl halide, it will react faster than regular alkyl halides
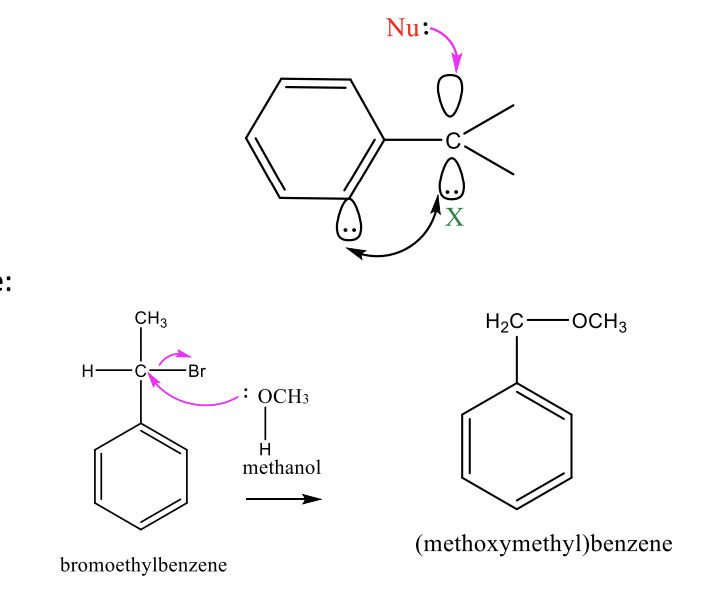
Sn2 Reaction with Benzene
One step simple nucleophilic substitution
leaving group is replaced by strong non-bulky nuc
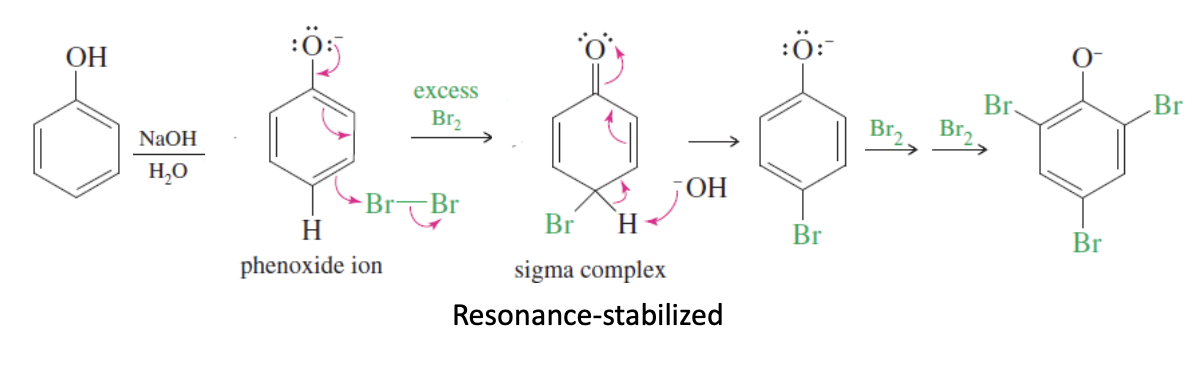
Phenol Reactions
2 types: phenol acylation and phenoxide ion formation
Phenol acylation: phenol reacts with acetic acid, producing esters
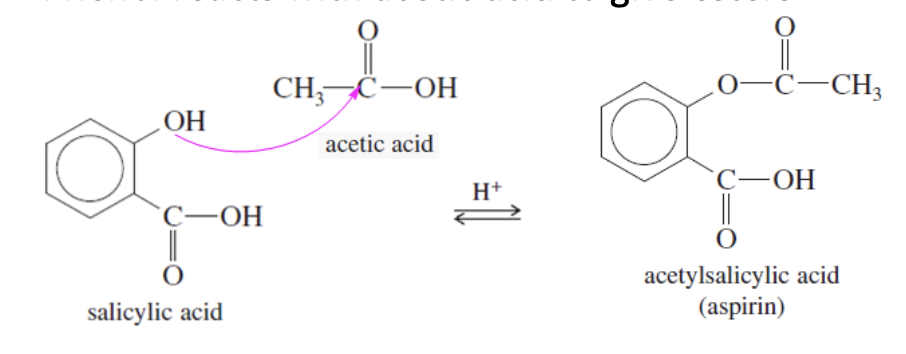
Phenoxide ion formation: Phenol reacts with NaOH to give phenoxide ions
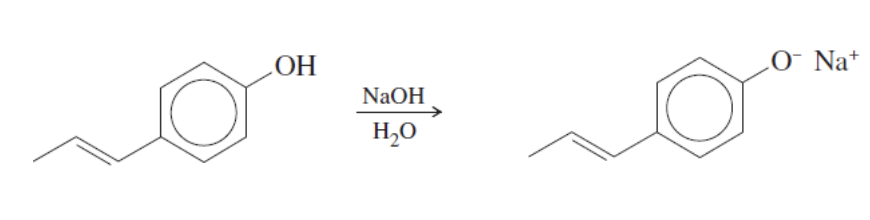
Phenol Electrophilic Aromatic Substitution
Phenol —NaOH, H20 —> Phenoxide Ion Formed —> +Br2 —> sigma complex —> 2 Br2 —> tribromo phenol
ortho and para directing