DDT - Toxicology (Peak)
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
What is pharmacokinetics and 4 stages of pharmacokinetics?
How the body interacts with administered substances in the body. Absorption (how the drug enters the body), distribution (how the drug spreads through out the body), metabolism (how the drug is processed in the body) and excretion (how the drug is removed from the body) are the 4 stages.
Factors of absorption when administering drug?
Route of administration (Oral, intravenously, inhalation, sublingual, buccal), how the drug behaves and environment in the body like pH, blood flow.
4 mechanisms of absorption of drugs in GI
Passive diffusion, facilitated diffusion, active transport and endocytosis
What is bioavailability?
Rate and extent drugs reaches the systemic circulation system. Route of administration can greatly affect bioavailability
How are drugs filtered by kidneys (glomerular filtration)?
Drugs enter through renal artery to glomerular capillary plexus. Filtered by kidney, it goes through bowman’s capsule. Done by small drugs, not bound to albumin (proteins)
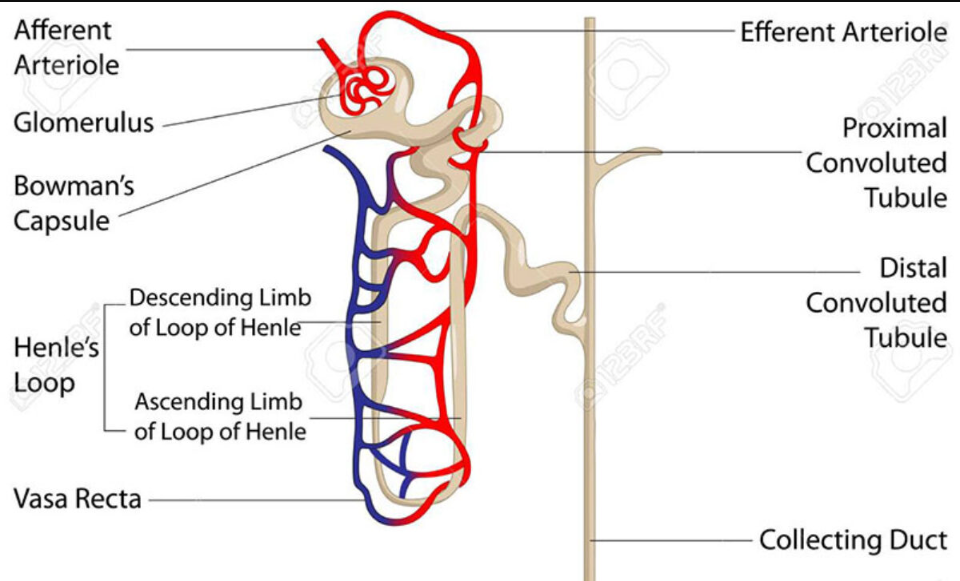
How are drugs filtered by kidneys (Active tubular excretion)?
If drugs have not been filtered, 2 active transport channels secrete anions and cations to get drugs to enter the proximal tubule. Competition between different drug can happen during this time
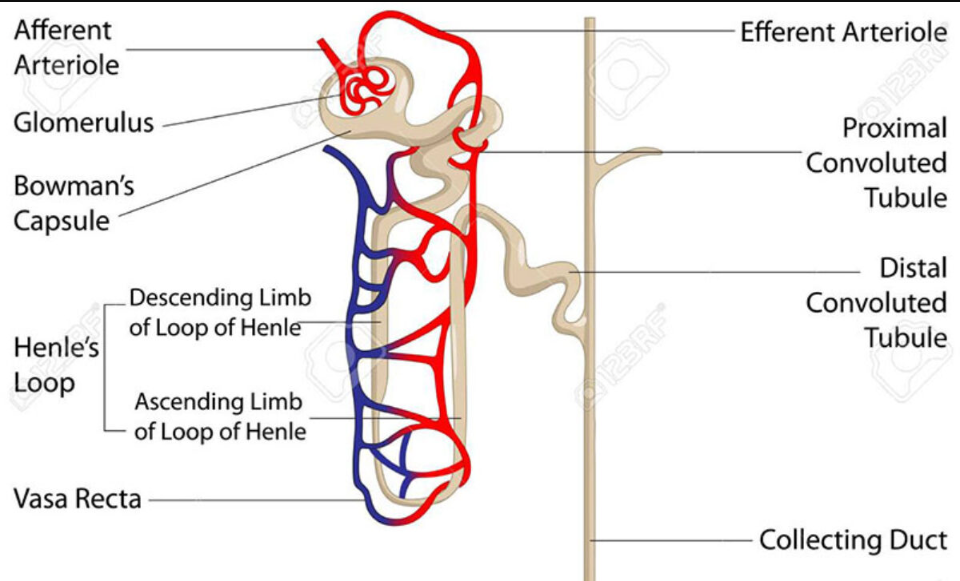
How are drugs filtered by kidneys (Distal tubular reabsorption)?
As drugs get to the proximal tubule, concentration of drugs increase, and some may be uncharged, so urine pH is manipulated to cause some drugs to be ionised, to prevent reabsorption, so it can be completely excreted
What is therapeutic drug monitoring
Measuring drug levels so it doesn’t become too toxic for the patient, during treatment
What are the 2 phases (and subphases) of xenobiotic metabolism in the liver
Phase I consists of hydroxylation (adding OH onto drug), reduction (electrons are added to drug to become more polar), oxidation (electrons are now removed?) and hydrolysis (breaks down drug using water into two component). Phase I essentially breaking the drugs into metabolising
Phase II consists of conjugation (where the drug is ionised), becoming easier to excrete
What is cytochrome p450 and Reactive Oxygen Species?
A monooxygenase (enzymes which add 1 oxygen into substrate), that adds oxygens to toxic molecules. Reactive oxygen species can be a by-product of the reaction, which are unstable, and are removed by enzymes and anti-oxidants.
What is pharmacodynamics?
How drugs act on the body and the effects they can cause, either by activating or blocking receptors or enzyms.
Different drug targets?
Ion channels (affects ion channel behaviour, either blocking them or opening them), G-protein coupled receptors (affecting enzyme activity and DNA transcription), enzymes (either blocking enzyme or give mimic substrate to enzyme, or create an active drug) and carriers/transporters (can block the transport of molecules or lead to fake compound)
Potency, efficacy, EC50, affinity and Kd definitions
Potency: Determines effectiveness of drug binding to receptor (higher potency = lower conc needed for effect to occur(
Efficacy: Limit before drug cannot cause any more effects, regardless of concentration given
EC50: Same as efficacy but limit is instead 50%
Affinity: How well drug can bind to receptor
Kd (dissociation constant): Where half the receptors have been binded and are being affected
Relationship between EC50 and Kd
If EC50 is higher than Kd, then spare receptors are present, which have not been binded to the drug
Relationship between affinity and Kd
Inversely proportional to affinity, so higher Kd = lower affinity, since less drug is required
Difference between agonists and antagonists?
Agonists bind to receptors, mimicking similar molecules, either by fully or partially activating the receptor. Antagonists just block the receptor, stopping a response from happening
Difference between full, partial and inverse agonist?
Full agonists produce maximum possible response when activating the receptor
Partial agonists produce a partial response when activating the receptor
Inverse agonists reduce the activity of the receptor
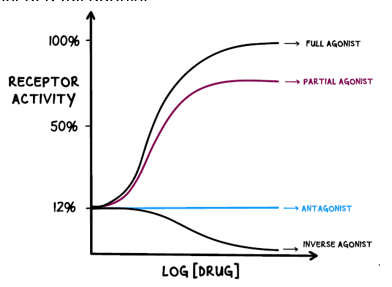
Difference between chemical, physiological and pharmacological antagonists
Chemical antagonists bind to another drug, rendering it useless, as it cannot bind to a receptor
Physiological antagonists produce a different effect to what another drug would have caused
Pharmacological antagonists bind to receptors, blocking them. It can either directly bind to the active site, or another site, which can also block an agonist
Types of pharmacological antagonists
Competitive: Binding to the same receptor against the agonist
Non-competitive: Binds to another site, causing permanent change to the agonist site, causing absolutely zero agonist effects
Ways to detect drugs
Liver function test: Testing ALP, AST, ALT, albumin and bilirubin levels to measure functions carried out by the liver
Mass spec: By ionising the samples, and separating the ions based on mass to charge ratio, the ions are detected which go through the data system to present the charge.
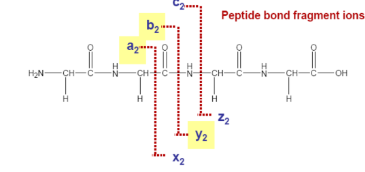
Where is the most common fragmentation likely to happen in this peptide bond?
At the b and y fragmentation area
If a compound is likely to degrade at high temperatures, would it be better to use GC/MS or LC/MS
LC/MS would be the better choice, as if the compound will degrade, accurate results cannot be obtained, like ketamine
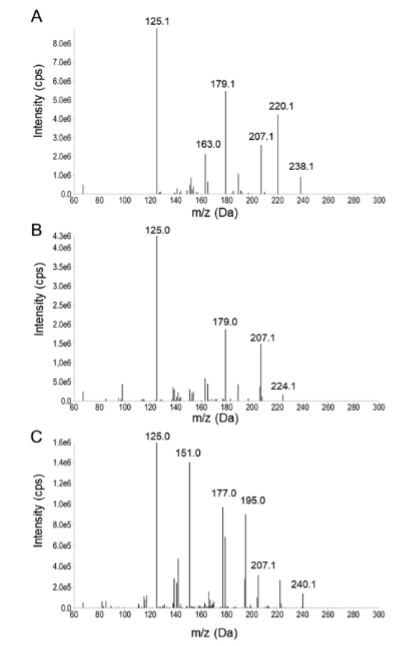
Ways to detect compound on mass spec graph
The last fragmentation typically shows the mass to charge ratio of the entire compound. Minusing 1 off this number will result in the molecular weight of the compound (238 would be ketamine, 224 would be norketamine and 240 cis-6-hydroxynorketamine, taking 1 off these resulting in the molecular weight for the compounds)