Hemoglobin and Iron Metabolism (Video Notes)
1/108
Earn XP
Description and Tags
Vocabulary-style flashcards covering Hb structure, synthesis, regulation, oxygen binding, disease-related variants, and iron metabolism concepts from the video notes.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
109 Terms
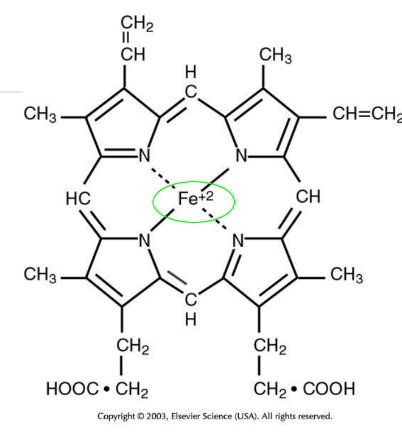
Heme
Protoporphyrin IX bound to iron (Fe); can reversibly bind one O2 molecule; gives blood its red color.
Hemoglobin (Hb)
Protein in RBCs composed of heme plus globin; accounts for about 95% of the RBC cytoplasm; Hb concentration is ~34 g/dL and contributes to RBC viscosity and deformability.
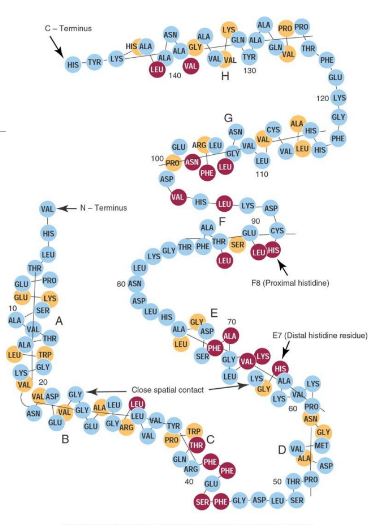
Globin
Protein portion of Hb consisting of four polypeptide chains (two pairs); each chain ~141–146 aa (alpha - theta); organized into eight helices (A–H) and seven nonhelical segments.
2 alpha chains
2 non alpha chains
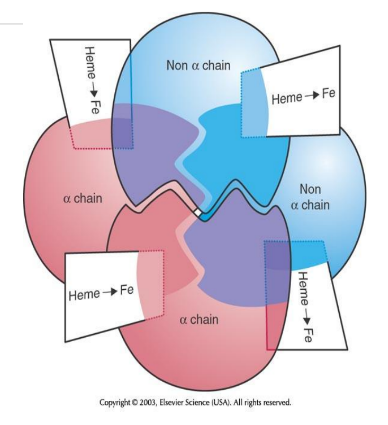
Complete Hemoglobin Molecule
Four globin chains form a pocket for four heme groups; each heme carries one O2, allowing Hb to carry up to four O2 molecules.
4 global, 4 heme+ 4 irons forms →
oxygen
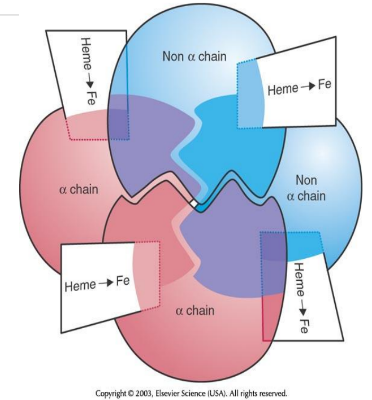
Heme Insertion Site in Hb
Heme is inserted at histidine residues on the E7 and F8 helices of the globin chains.
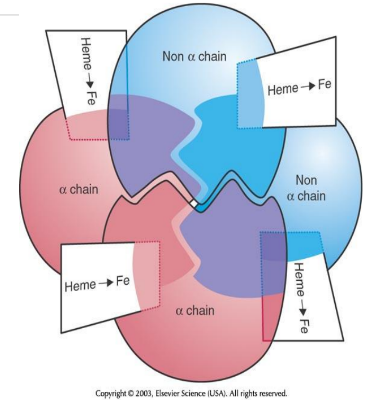
4 Heme + 4 Globin =
1 Hemogloblin
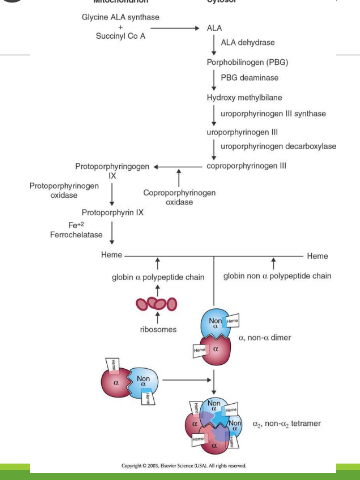
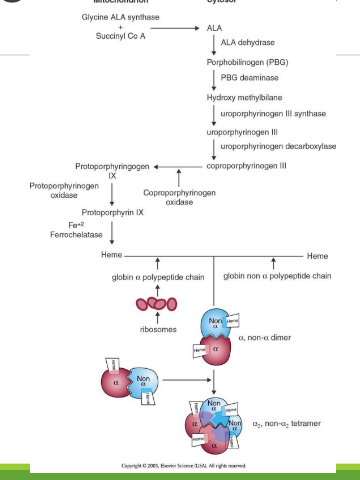
Heme Synthesis Location
Occurs in bone marrow erythrocyte precursors, in mitochondria and cytoplasm; part of Hb biosynthesis.
Heme Synthesis happens in erthrocyte precursor stages _____ to _______
pronormoblast to polychromatic erythrocyte (retic)
Globin Synthesis Location
Protein synthesis from mRNA in the cytoplasm of erythroid precursors.
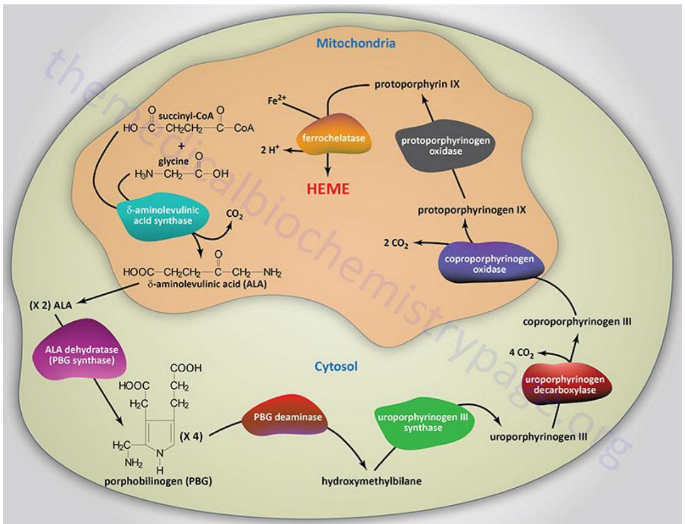
Synthesis of Protoporphyrins (Heme) Location
Begins in the mitochondria with the formation of delta-aminolevulinic acid (d-ALA) from glycine and succinyl-CoA
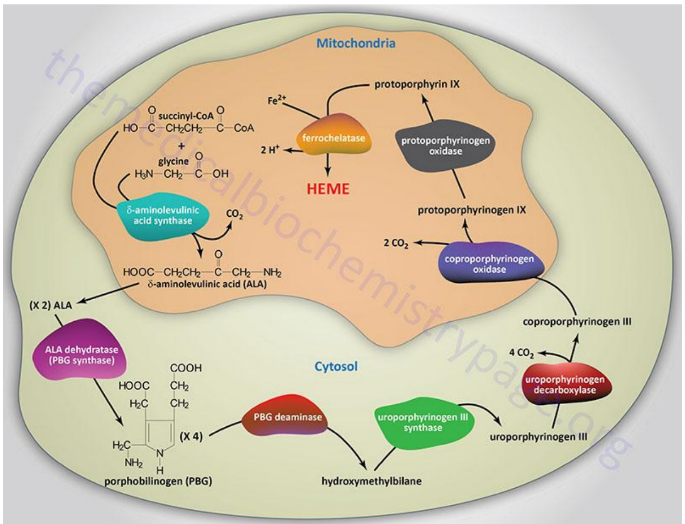
d-ALA Synthesis
is formed from glycine and succinyl-CoA in mitochondria; catalyzed by d-ALA synthase, the rate-limiting step of heme biosynthesis.
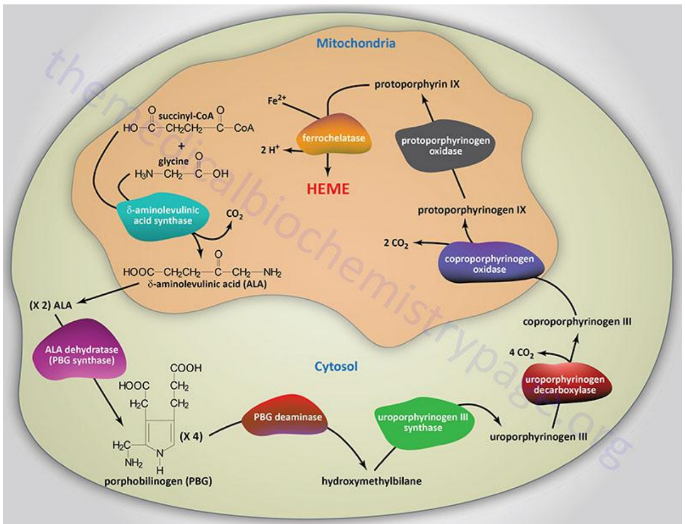
d-ALA Synthase
Enzyme that catalyzes the formation of d-ALA; activity is inhibited by vitamin B6 (pyridoxine) deficiency.
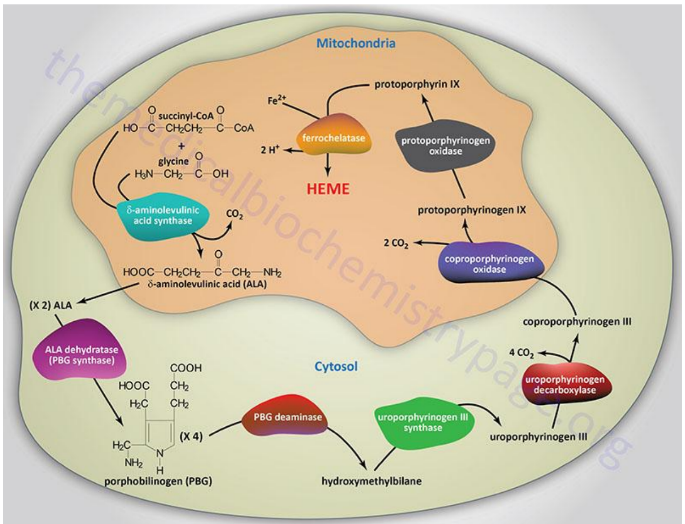
Porphyrinogens
Unstable, inactive forms of porphyrins that are intermediates in porphyrin/heme biosynthesis.
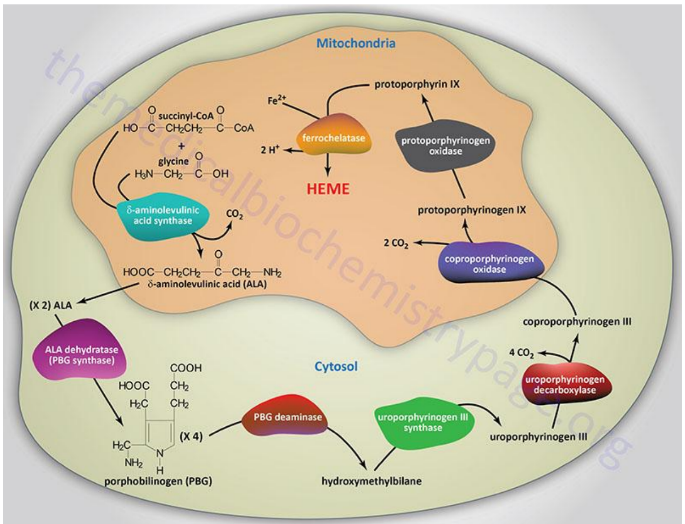
Porphyrias
Inherited defects in heme synthesis leading to accumulation of porphyrin precursors.
Iron Delivery to Erythroid Cells
Ferric (Fe3+) iron is transported in plasma by transferrin and is reduced to ferrous (Fe2+) iron inside cells for incorporation into heme.
True or False: Only ferrous iron can be incorporated into heme with ferrochelatase.
True
Ferritin
Storage form of iron; keeps iron in a readily available form; stores iron in liver and other tissues; increased in iron overload, decreased in deficiency.
Transferrin
Plasma protein that binds Fe3+ and transports iron to tissues, including RBC precursors.
Genes for Globlin Biosynthesis for chromosome 16
alpha and zeta
Genes for Globlin Biosynthesis for chromosome 11
beta, gamma delta, epsilon
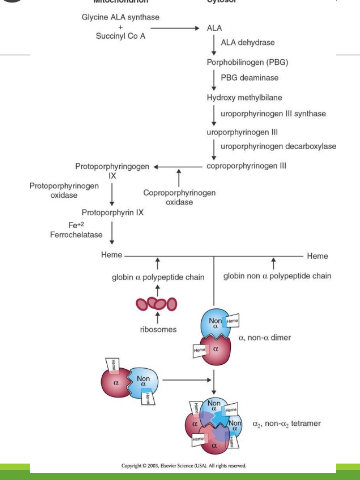
Hemogloblin A
The main form of hemoglobin in adult humans, composed of two alpha and two beta chains. It is responsible for oxygen transport in the blood.
Hemoglobin A2
A minor form of hemoglobin in adults, comprising two alpha and two delta chains, constituting about <3.5% of total hemoglobin.
Hemoglobin F
The primary hemoglobin found in the fetus, consisting of two alpha and two gamma chains, it has a higher affinity for oxygen than adult hemoglobin, constituting about 1-2% of total hemoglobin.
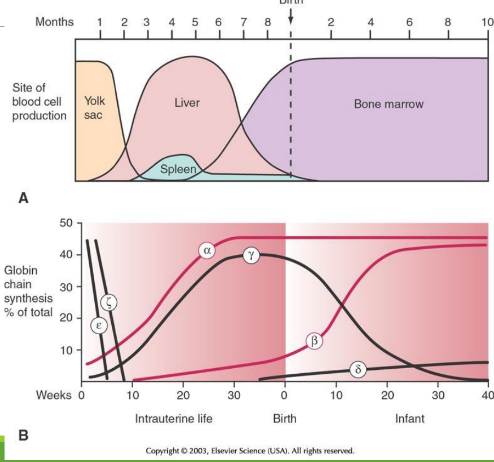
Embryology through childhood: week 0-10
epsilon and zeta are in egg yolk, but decrease
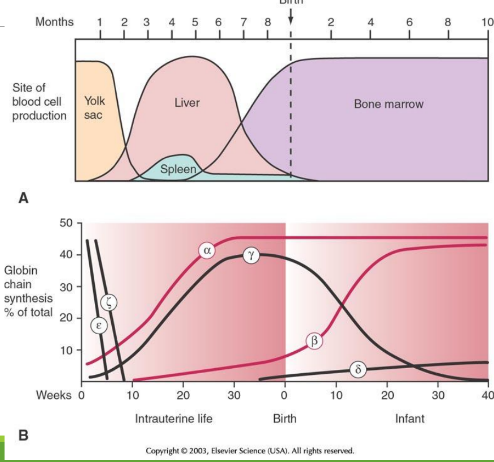
Embryology through childhood: week 10-week 0 at birth
during hepatic stage and medullary stage, alpha and gamma starts to increas by a lot, while beta increases slowly
Embryology through childhood: week 0 at birth-week 10 at birth
alpha stays consistent, while beta increases more at week 10. Delta then starts to increase slowly. At week 10 there is the beta-gamma switch
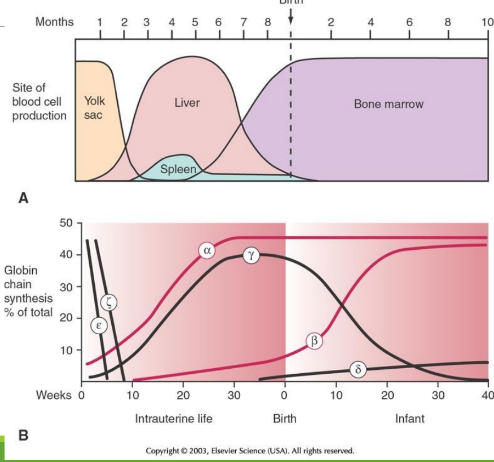
Embryology through childhood: week 10-week 0 at birth
alpha stays consistent, while beta increases more after week 10, predominating over gamma. Delta still increases slowly.
Globlin chains of Intrauterine (IU) in Gower 1
zeta + epsilon
Globlin chains of Intrauterine (IU) in Gower 2
alpha + epsilon
Globlin chains of Intrauterine (IU) in Portland
zeta + gamma
Embryonic/Perinatal Hb Forms
Intrauterine Hb includes Gower and Portland types; HbF predominates at birth; HbA becomes predominant in infancy.
Heme Regulation
Heme inhibits ALA synthase, ◦ Increase heme → decrease amount of ALA synthase
Globin Regulation
Transcription = Promoter + Kruppel-like factor 1 + other transcription factors + locus control region
Translation = inhibited by lack of heme; increased in presence of heme
Hemoglobin Regulation
◦ Normally stimulated by tissue hypoxia
◦ Increased EPO release
Hemoglobin Functions in High Affinity
Bind oxygen readily in the lungs
Hemoglobin Functions in Low Affinity
Unload oxygen in the tissues
Affinity is dependent upon ______
partial pressure of oxygen (pO2)
Higher Partial pressure of oxygen →
higher affinity
Lower Partial pressure of oxygen →
lower affinity
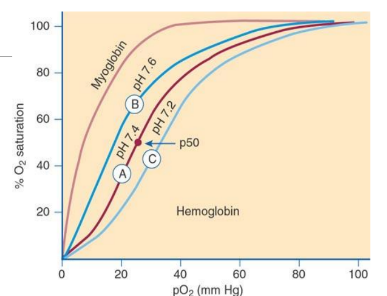
Bohr Effect
Oxygen affinity of Hb is modulated by pH; higher pH (alkalosis) increases affinity (left shift), lower pH (acidosis) decreases affinity (right shift).
Right Shift in Oxygen Dissociation Curve
increase of H+ ions, lower blood pH, higher 2,3 BPG, higher pCO2, higher temperature, lower oxygen affinity → decrease in oxygen affinity
Left Shift in Oxygen Dissociation Curve
decrease of H+ ions, higher blood pH, lower 2,3 BPG, lower pCO2, lower temperature, higher oxygen affinity→increase in oxygen saturation
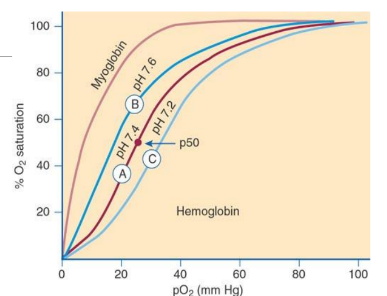
Oxygen Dissociation Curve
Plot of Hb oxygen saturation versus pO2; indicates how readily Hb binds/releases O2. Shifts to the left/right is dependent on blood pH (Bohr Effect)
P50
Partial pressure of O2 at which Hb is 50% saturated; normally ~27 mm Hg; lower P50 means higher affinity, higher P50 means lower affinity. ph 7.4
Deoxyhemoglobin
specific spatial configuration that resembles an egg with a central cavity; has 2,3-DPG keeping the structure close together
Oxyhemoglobin
changes overall configuration which facilitates additional binding of oxygen. has no 2,3-DPG
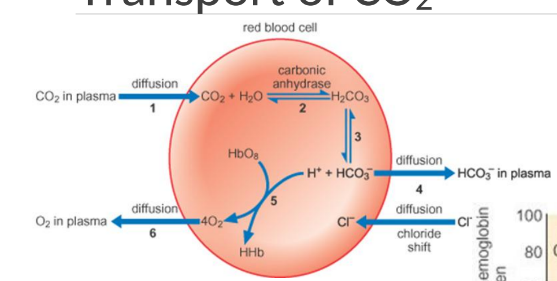
Increase of CO2 during transport of CO2 across membrane
Co2 diffuses across the membrane into red blood cells, promoting the release of oxygen from hemoglobin and enhancing the production of bicarbonate.
Bohr effect in transport of CO2
has a right shift in the oxygen-hemoglobin dissociation curve, meaning that increased levels of CO2 lower the affinity of hemoglobin for CO2, facilitating oxygen release to tissues.
What does chloride do in transport of oxygen?
Chloride shifts into red blood cells to balance the negative charge as bicarbonate ions diffuse out, facilitating the transport of oxygen and influencing hemoglobin function.
Globin Gene Clusters
Alpha-like globin genes located on chromosome 16 (e.g., zeta, alpha); beta-like globin genes on chromosome 11 (e.g., epsilon, gamma, delta, beta).
CO2 Transport
CO2 is transported in plasma and as bicarbonate; carbonic anhydrase catalyzes CO2 + H2O to H2CO3, then HCO3-
Methemoglobin
Hb with Fe3+ (ferric) that cannot bind O2 with spectrometry detection at 630 nm; Toxic levels: 1.5g%
Why is methemoglobin a problem?
Fe3+ cannot bind to oxygen → left shift in the oxygen dissociation curve, leading to reduced oxygen delivery to tissues → cyanosis
What color the blood would be for methemoglobin?
Dark chocolate brown
Can methemoglobin be corrected?
yes through Oxygen inhalation and Strong reducing substances
Sulfhemoglobin
Hb modified by irreversible oxidation from certain drugs/chemicals (e.g., sulfonamides); spectrometry detected at 630 nm’ toxic level: 0.5%
Why is sulfhemoglobin a problem
causes a left shift in the oxygen-hemoglobin dissociation curve → cyanosis
Can sulfhemoglobin be corrected?
No, it is irreversible.
Carboxyhemoglobin
heme iron bound to carbon monoxide (CO); spectrometry detected around 540 nm; Toxic level: 20-30% (cherry red color)
Reference Ranges for Carboxyhemoglobin
<3% (nonsmokers); 4-15% (smokers)
Why is carboxyhemoglobin a problem?
CO has an affinity 240x greater than oxygen. CO binds Hgb and prevents oxygen from binding thus leading to incredible cyanosis and massive tissue necrosis
Can carboxyhemoglobin be corrected?
Yes, it can be corrected with oxygen therapy or by removing the source of carbon monoxide.
Dietary Iron Sources
Heme iron from animal products (e.g., Hb, myoglobin) and nonheme iron from legumes and leafy vegetables; heme iron often better absorbed.
Average diet for dietary iron
Average diet: 10-20 mg/day
◦ Actual absorption: 1-2 mg/day
◦ Sufficient for most men, but others may need more
Iron is absorbed in two forms:
heme and nonheme iron.
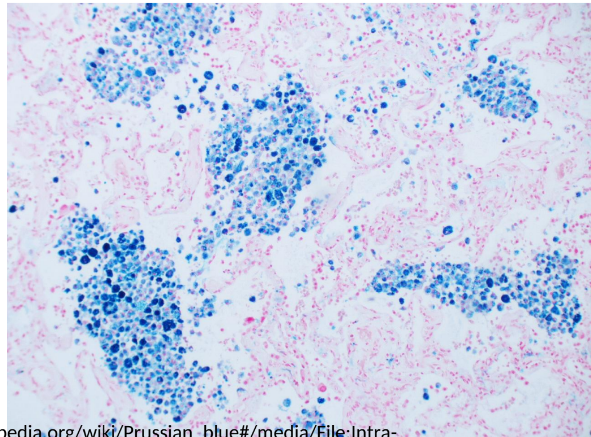
Prussian Blue (Iron Stain)
Histochemical stain used to detect iron in tissues; gold standard for assessing iron stores in bone marrow.
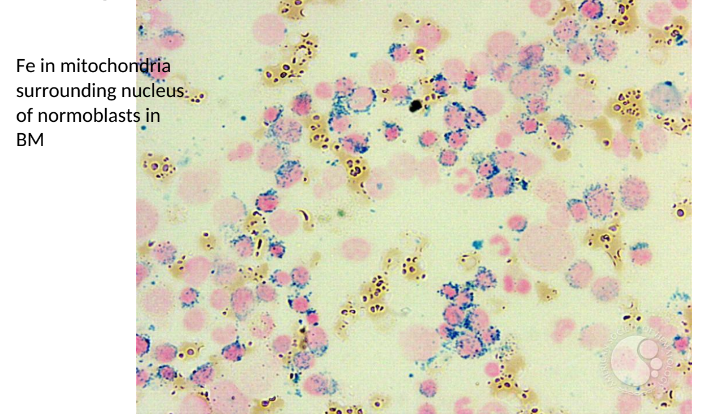
Ring Sideroblasts
Nucleated erythroblasts with iron-laden mitochondria forming ringed structures around the nucleus, seen with Prussian blue staining.
Serum Ferritin
Storage form of iron in macrophages and secreted by macrophages and can be measured in immunoassay; reference rangeL 40-400 ng/mL
Clinical Significance in Low levels of Ferritin
iron deficiency anemia
Clinical Significance of Acute phase protein in Ferritin
hard to determine deficiency
Clinical Significance in High levels of Ferritin
organ damage, inflammation, pregnancy
TIBC (Total Iron Binding Capacity)
Total available iron-binding sites on transferrin; reflects transferrin concentration; normal range ~250–400 µg/dL; can be used with serum iron to calculate transferrin saturation.
In a normal, healthy
individual, iron occupies _____ of the binding sites on transferrin
20-50%
Serum Iron
Amount of ferric iron bound to transferrin in serum = availability of transport iron; typical reference range 50–160 µg/dL; analyzed in Chromogen-Spectrophotometric Methods
Special considerations for serum iron
◦ Does not include iron in hemoglobin.
◦ Diurnal variation: highest in the morning
◦ Limited utility on its own
% Transferrin Saturation
% of transferrin binding sites that are iron-bound
Calculation of % Transferrin Saturation
[Serum Iron/TIBC] x 100
◦ TIBC = total # of sites for Fe binding
◦ SI = # bound with Fe
Why is % Transferrin Saturation a better diagnostic indicator for disease?
It establishes a relationship
between iron and transferrin.
◦ High in Fe overload; low in Fe deficiency
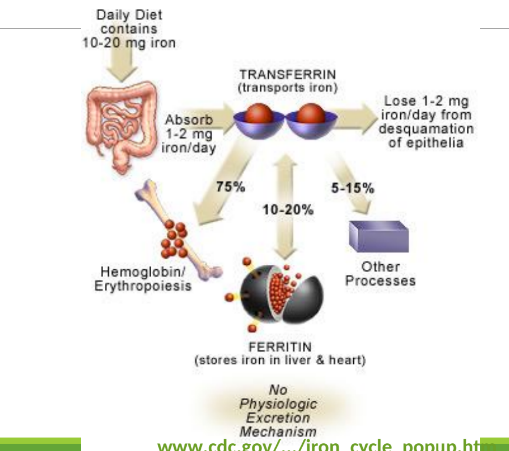
Distribution of Iron
Transferrin bound ot iron will give to iron in:
75% hemoglobin/erthropoeisis
10-20% in ferritin when it stores in liver and heart
5-15% for myoglobin and other enzymes.
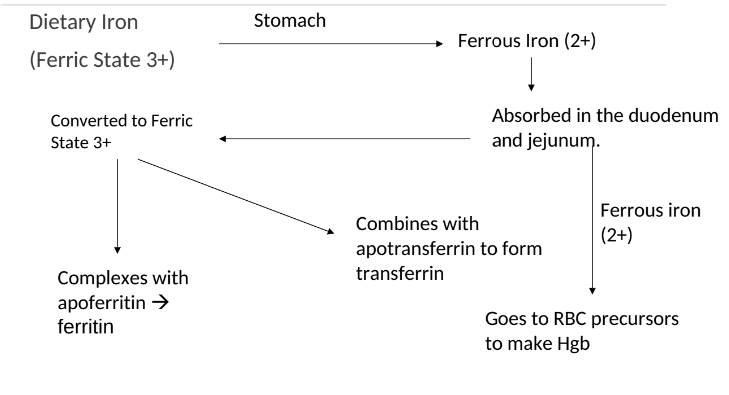
Iron Absorption
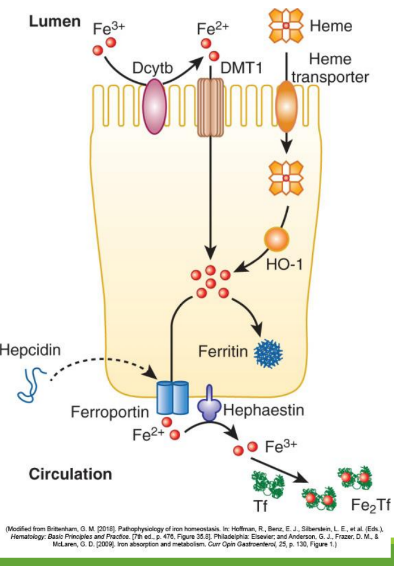
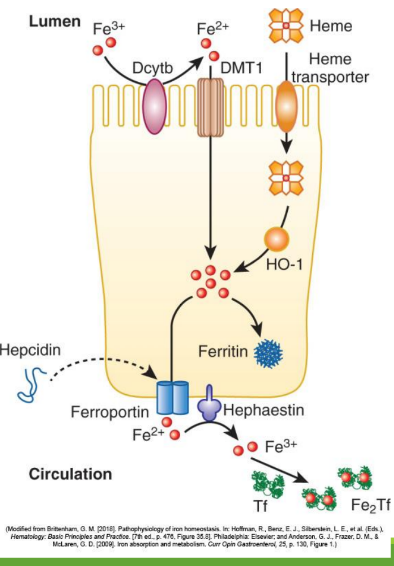
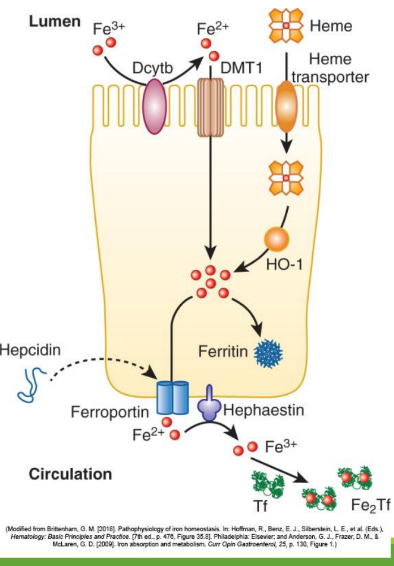
Dietary iron (ferric state 3+) goes through stomach converting to ferrous iron (2+). It is then absorbed in the duodenum and jejunum of the small intestine. From there, it is converted to ferric state (3+) to ferritin or transported by transferrin in the bloodstream. It can also stay as ferrous iron (2+) to go to RBC precursors to make hemoglobin.
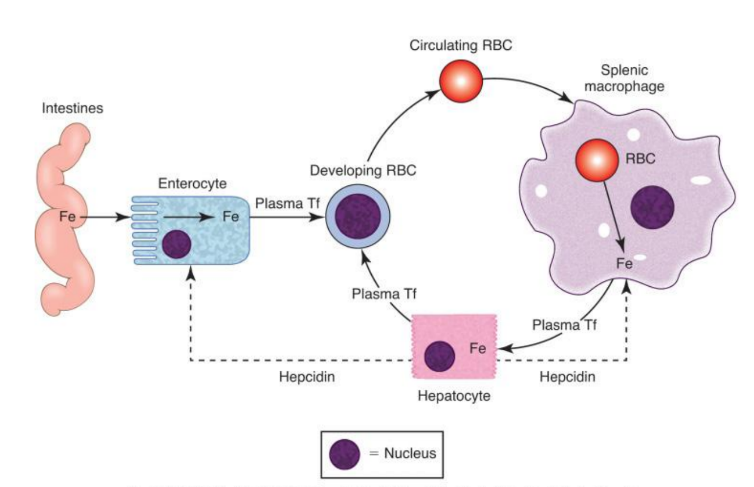
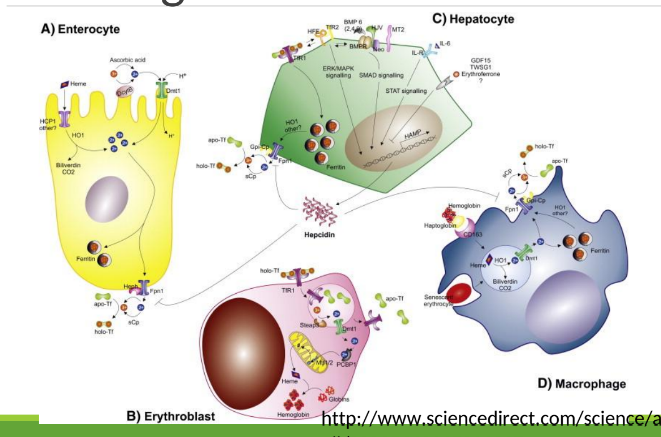
Enterocyte Iron Handling
In the gut, Fe3+ from the diet is transferred into entereocyte to plasma. It is then reduced and transported by DMT1; ferroportin exports Fe2+ into blood; hepcidin regulates this export.
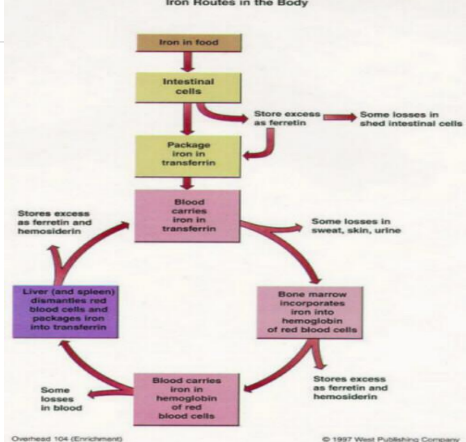
Iron Route in Body after iron is in transferrin
Blood carries iron in transferrin. It goes into bone marrow where it is utilized for hemoglobin synthesis in red blood cells, and excess iron is stored in the liver and spleen as ferritin.
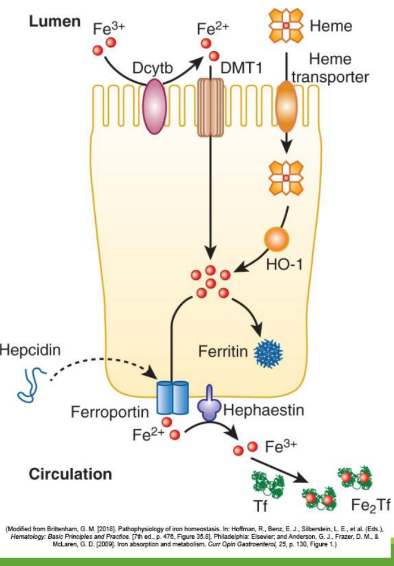
Divalent Metal Transporter 1 (DMT1)
A transporter protein that facilitates the uptake of ferrous iron (Fe2+) and other divalent metals into enterocytes from the intestinal lumen.
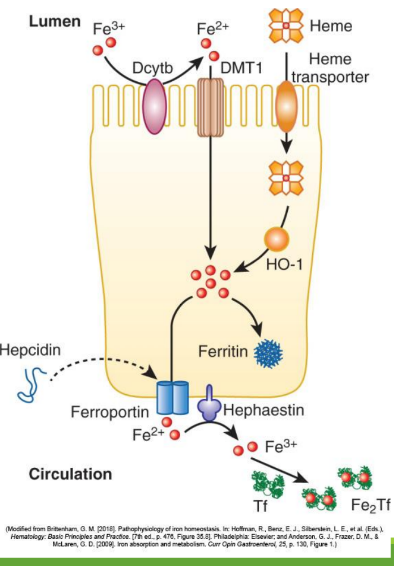
With excess Fe, liver makes ____ that binds to ferroportin preventing Fe+2 binding
hepcidin
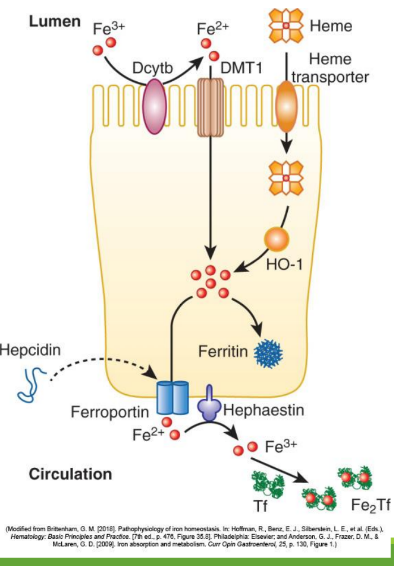
Hephaestin
in enterocyte membrane oxidizes Fe+2 to Fe+3
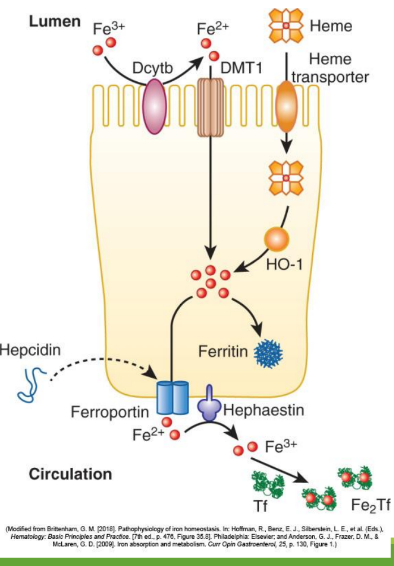
In blood Fe+3 (x2) binds to apotransferrin→ ____
transferrin
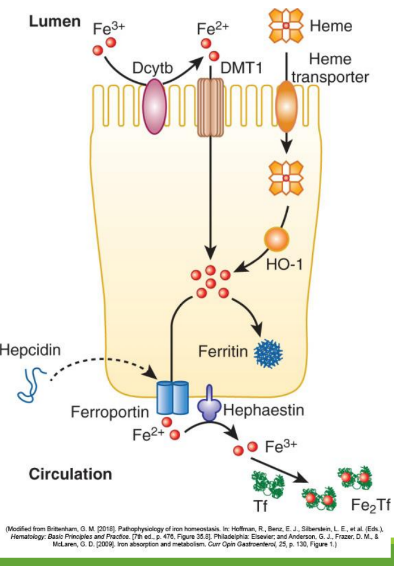
Transferrin Receptor (TfR1)
Cell-surface receptor that binds transferrin and mediates iron uptake by endocytosis into cells.
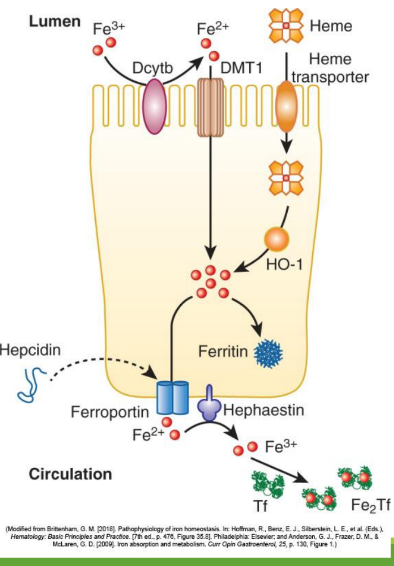
DMT1 _______ out of endosome into cytoplasm
moves Fe
Fe+3 binds to apoferritin =
ferritin
How many Fe3+ can 1 ferritin bind?
400
_______ and _______ store most Fe+3
macrophages, hepatocytes
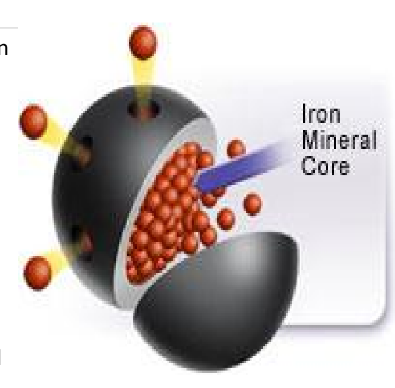
Apoferritin
protein shell sans iron
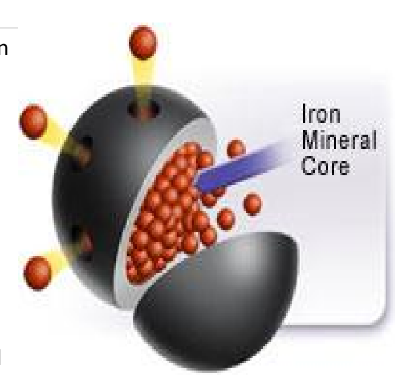
Hemosiderin
amalgam of degraded iron (micelle form) and a piece of the ferritin shell
Two forms of Recycling Iron
Extravascular hemolysis
Intravascular hemolysis
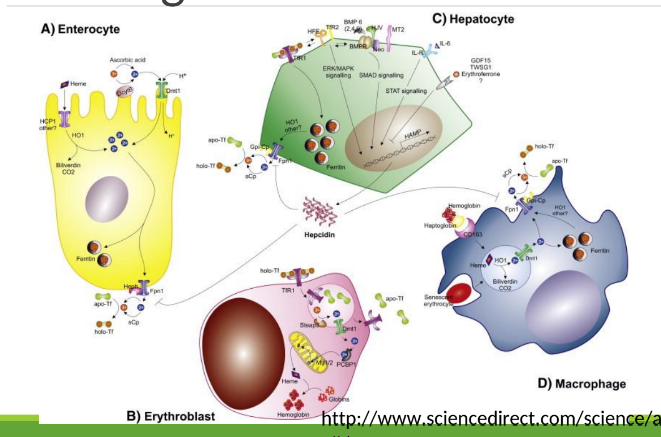
Extravascular hemolysis
Macrophage ingests RBC, degrades Hgb releasing Fe which binds to ferritin
Macrophages have ferroportin to export Fe to other cells
Macrophages in the spleen and liver recover iron from hemoglobin and return it to circulation via ferroportin.
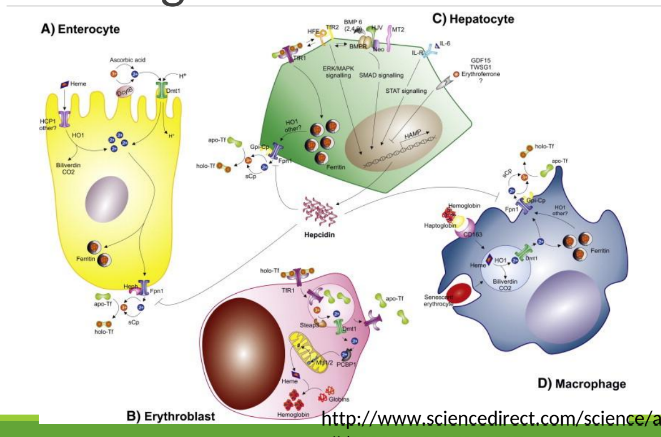
Intravascular hemolysis
◦ Haptoglobin/hemopexin in blood bind to Hgb/heme released from lysed
RBCs
◦ Hepatocytes have ferroportin too
Practical Iron Deficiency Definition (WHO)
Iron deficiency defined by ferritin <15 µg/L or transferrin saturation <16% or Hb rise after 2 months of iron therapy.
Acute-Phase Considerations in Iron Studies
Ferritin can be elevated with inflammation; timing and CRP should be considered when interpreting iron studies.
Iron Studies Laboratory Assessment
Serum Iron (SI)
Serum Total Iron Binding Capacity (TIBC)
Ferritin
Percent Transferrin Saturation
Prussian blue staining