Isomerism and Chirality
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Isomers:
different molecules but same atomic composition
Stereo-isomers:
molecules with same groups and bonding, but different spatial organisation
Chirality
is due to stereo-isomers ; this is the ‘handedness of molecules’
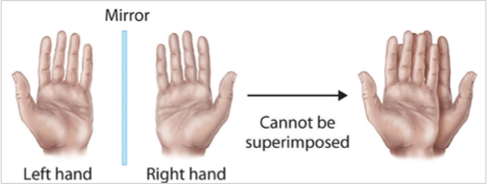
just look at this
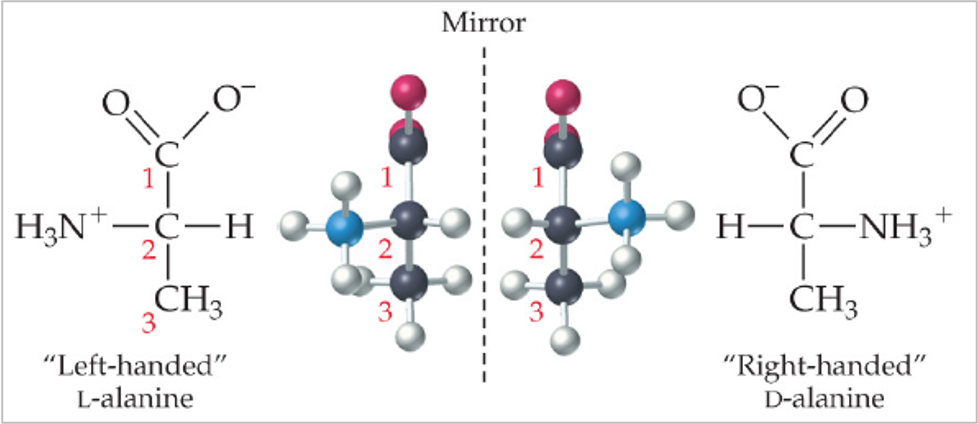
•Chiral molecules have the same MOLECULAR formula
They have left- and right-handed isomers called enantiomers
•All the amino acids used to make your proteins are L-isomers
•Enzymes and receptors will recognise a specific enantiomer of a chiral molecule
•GluR respond to L-glutamate but not D-glutamate
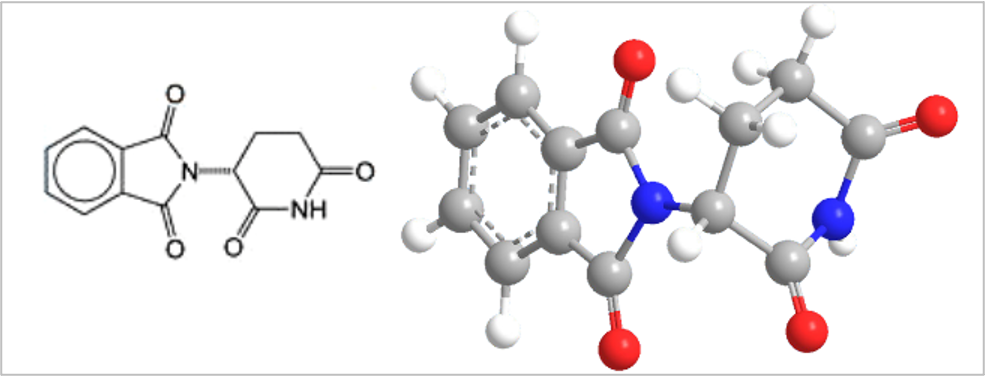
Thalidomide is a chiral molecule: has (R)- and (S)- enantiomers
•(R)-enantiomer acts as a sedative and was used to treat morning sickness.
•(S)-enantiomer acts as a teratogen causing birth defects. It is estimated that worldwide 10000 infants were born with limb malformation and other problems.
•The enantiomers can inter-convert in vivo.
•This tragedy led to more stringent drug testing and approval regulations.