Organic Chem pka values
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
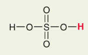
H2SO4 Sulfuric Acid
Pka = -9
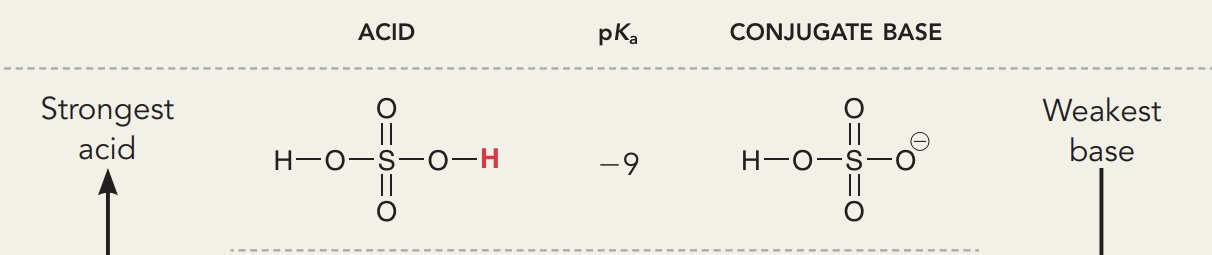
Protonated carbonyl, ketones, aldehydes, esters
Pka = - 7

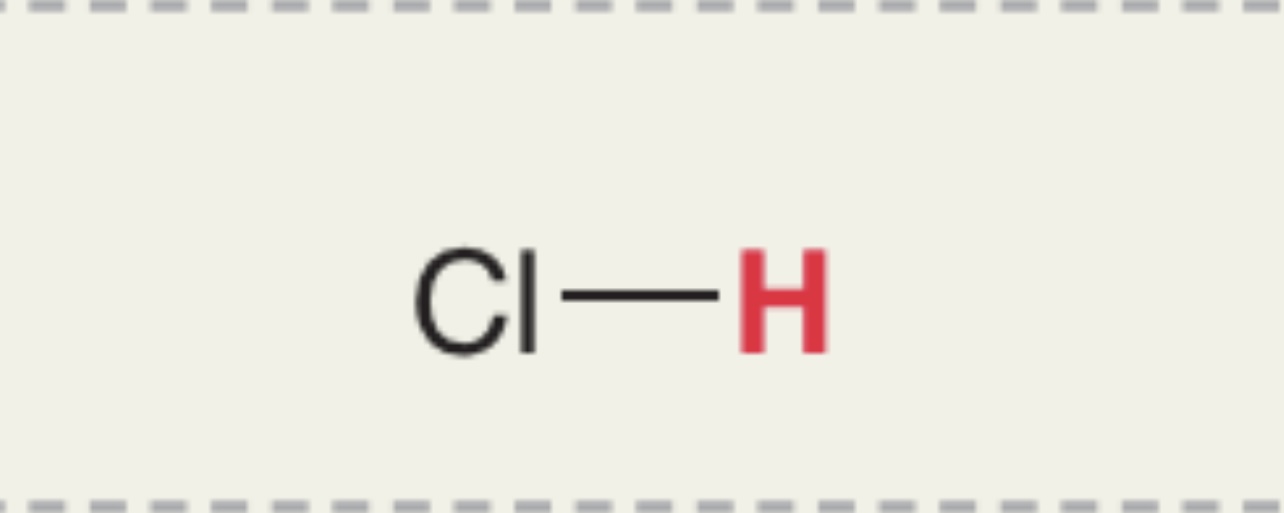
H in HCl
Pka = -7
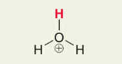
H in H3O+
Pka = -1.74
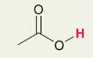
H in -COOH carboxylic acid
pka = around 5
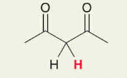
H in C - C2O2 , 2 carbonyl group
Pka = 9
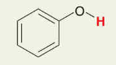
H in Phenol group ( a ring and -OH )
Pka = 10
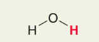
H in water H2O
Pka = 15.7 or 16
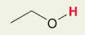
H in -OH alcohol linear carbon chain
Pka = 16
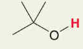
H in -OH with substitutued C or branch
Pka = 18
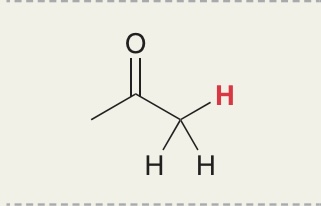
H in keton- CH3
Pka = 19
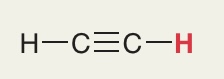
H in Alkyne triple bond
Pka = 25
H in NH3
Pka = 38
H in C2H4 alkene
Pka = 44
H in -C2H6 alkane
Pka = 50
H-H
pka = 36
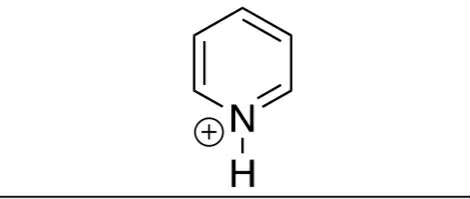
H in ring-NH(+)
pka = 6
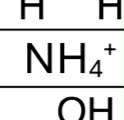
H in NH4(+)
pka = 9
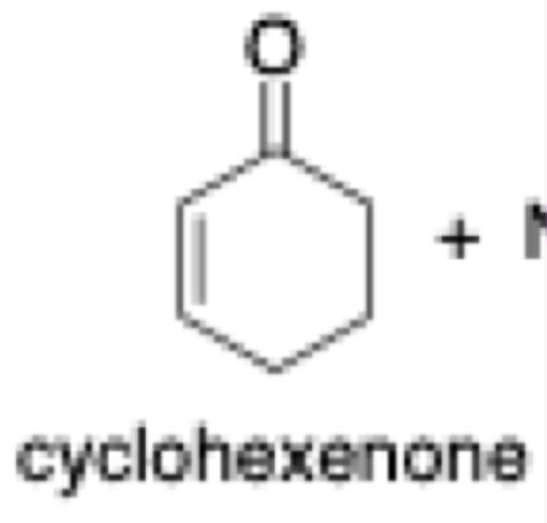
Cyclohexenone
pka = 16.7 - 20
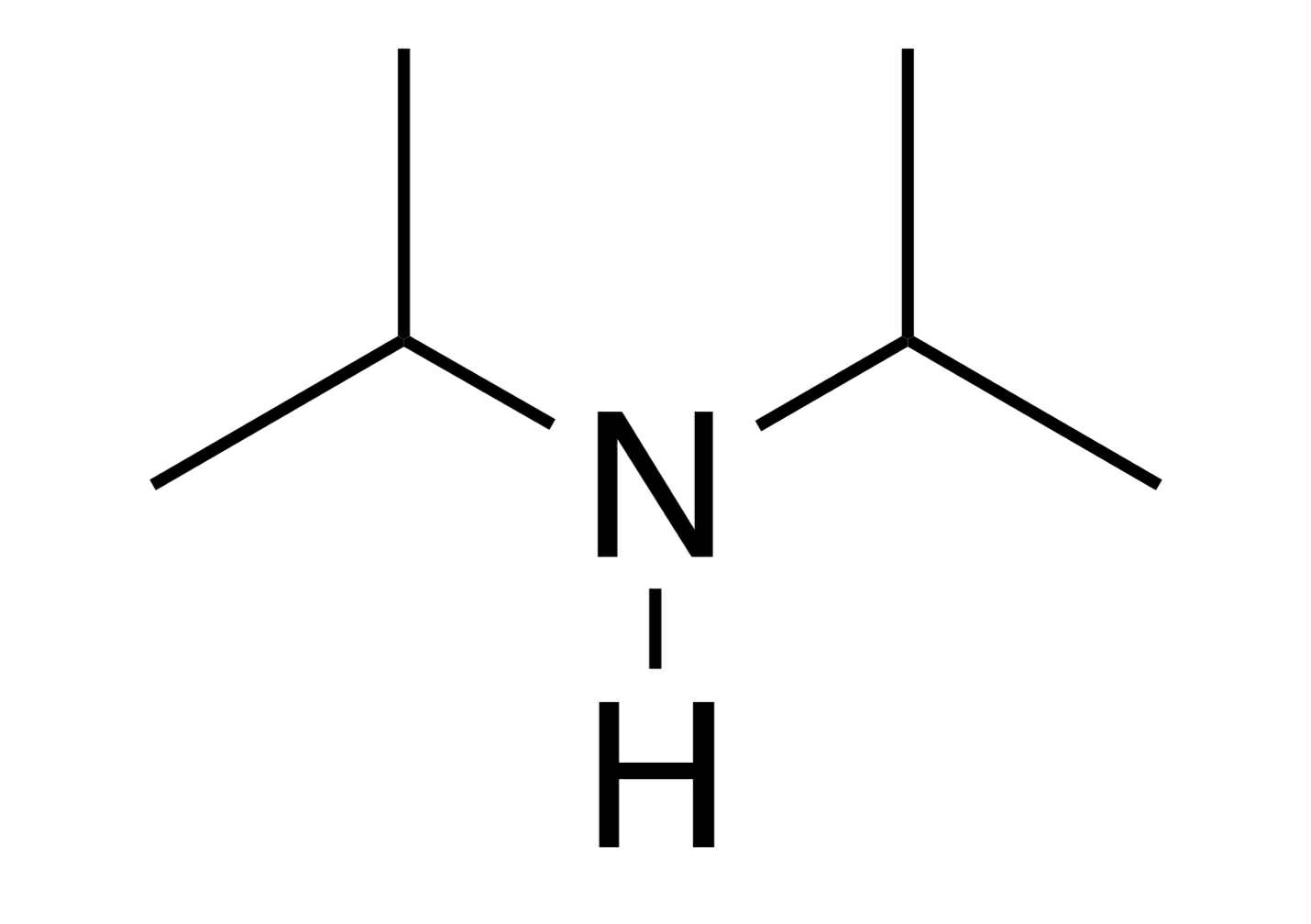
pka = 38
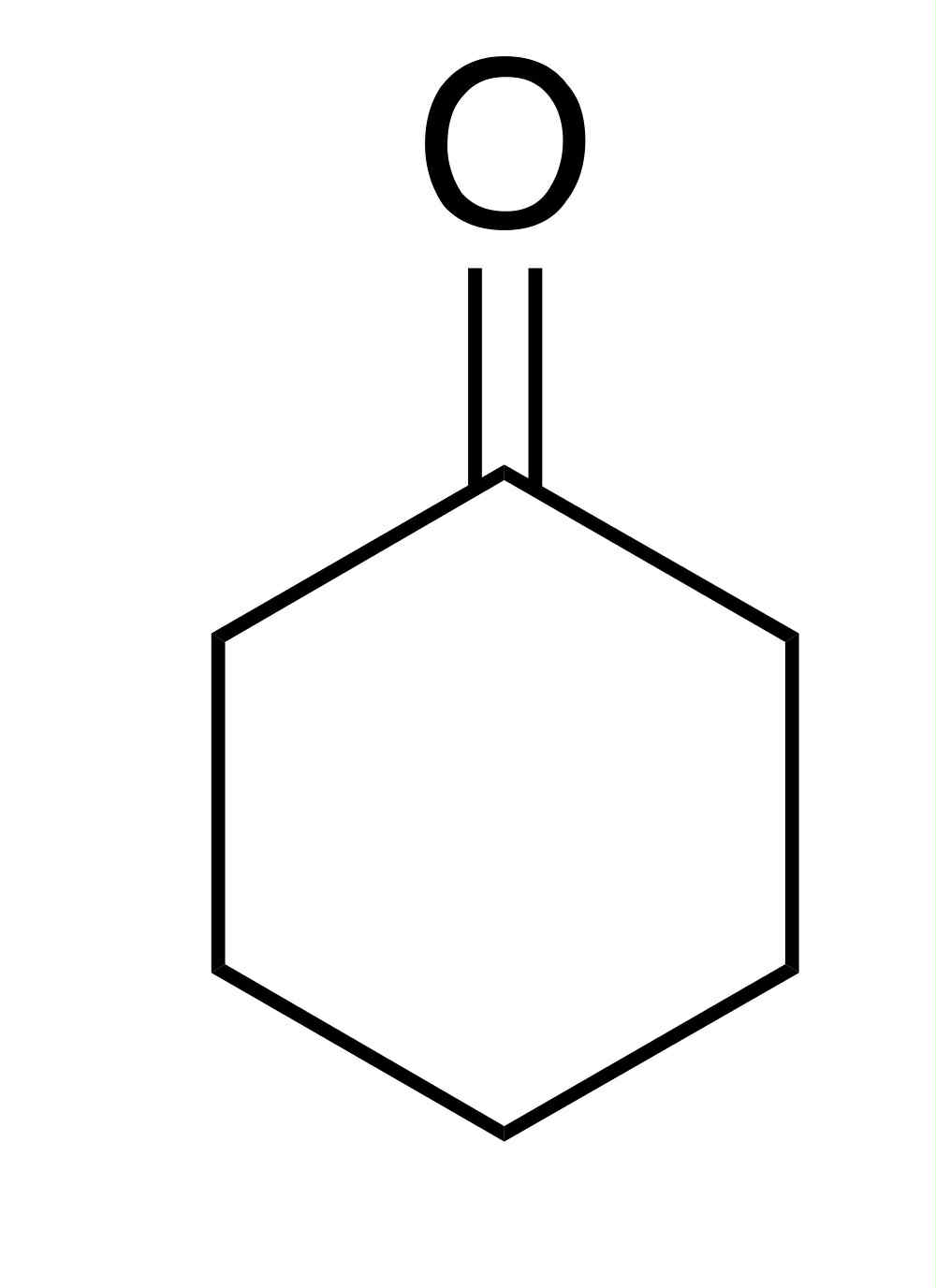
Cyclohexanone
pka = 20-24
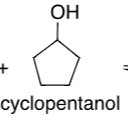
Cyclopentanol
pka = 16
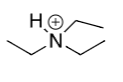
pka = 9
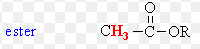
Ester
pka = 25
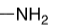
-NH2 , amine proton
pka = 38