Purity & mixtures: Elements compounds and mixtures: Chemistry: (9:1)
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Pure substance
Either a single element or a single compound
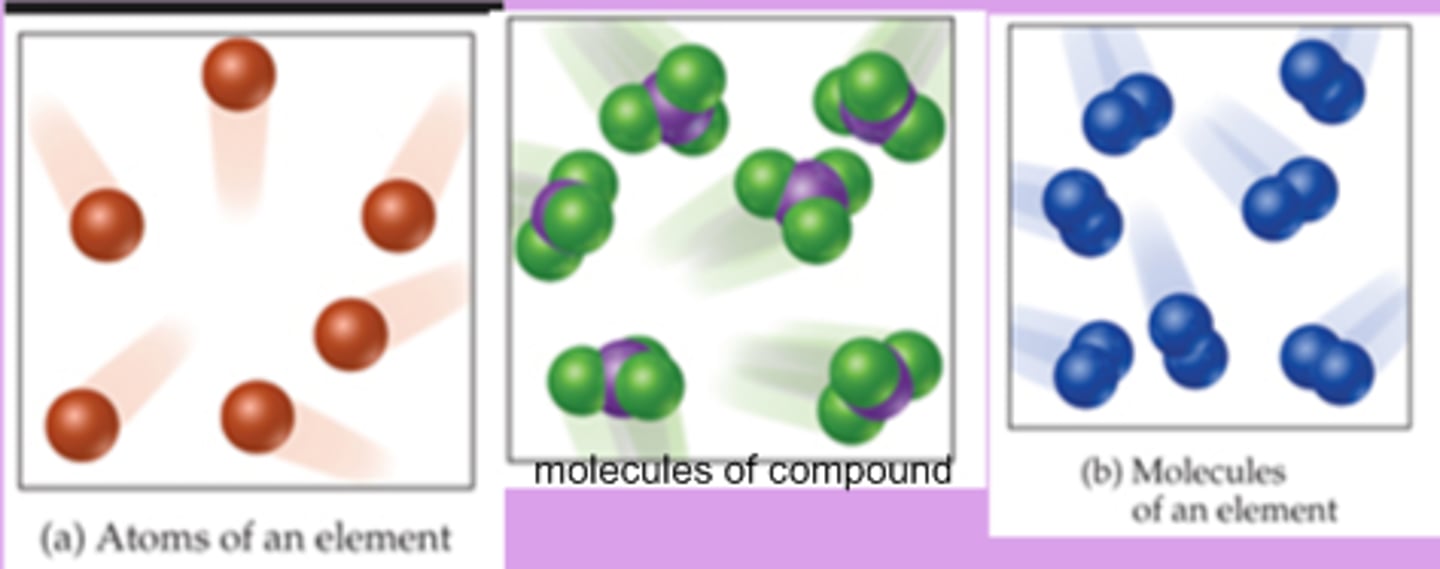
Mixture
A combination of two or more substances that are not chemically combined
Formulation
A mixture that has been designed for a specific purpose

Melting/boiling point of pure substances
A single defined temperature
Melting/boiling points of mixtures
A range of temperatures
Melting point of pure water
0 °C

Boiling point of pure water
100 °C

Examples of formulations
Paint, processed food, fuels, cleaning products, cosmetics

Test for purity
Test melting/boiling point
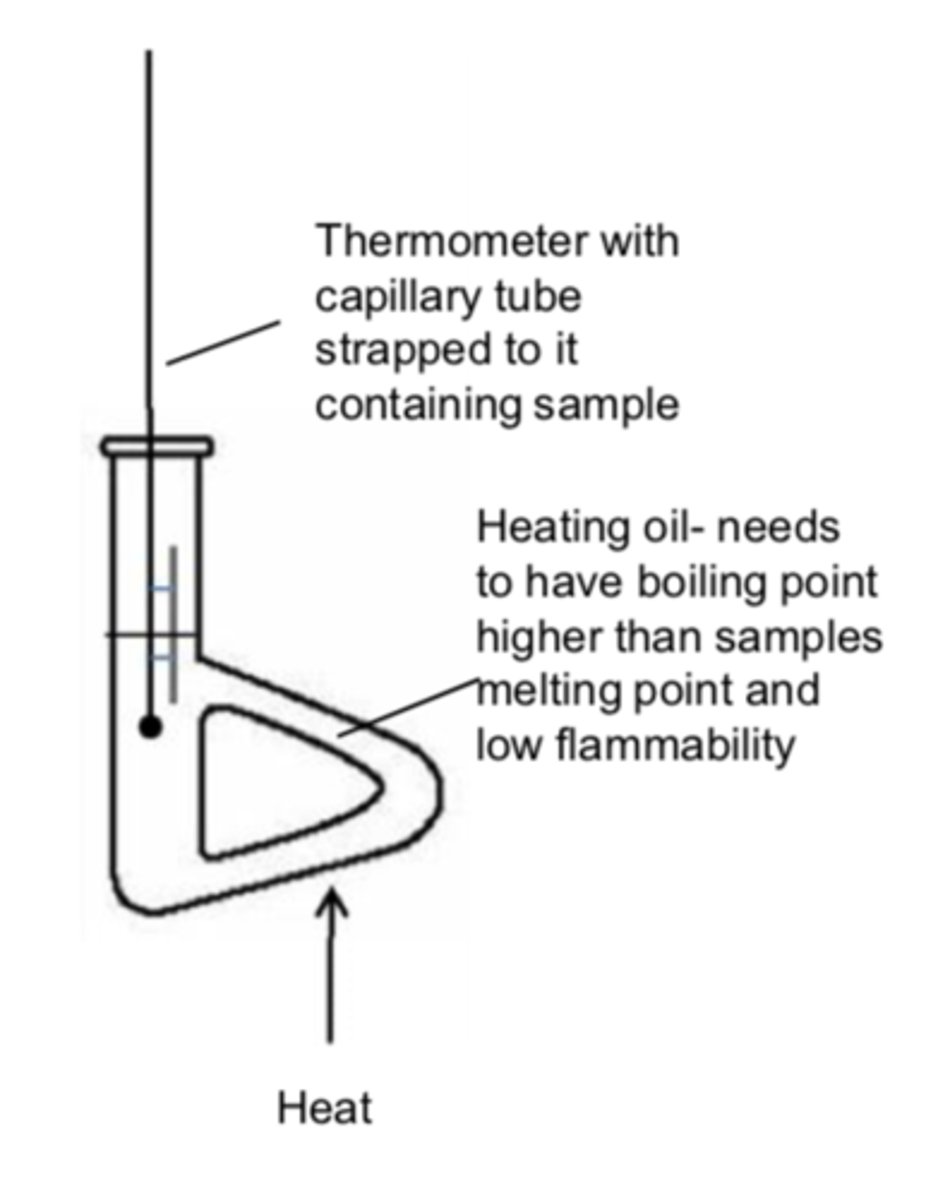
Test if water is pure
Test if boiling point is exactly 100 °C
Solution
A mixture that forms when one substance dissolves another
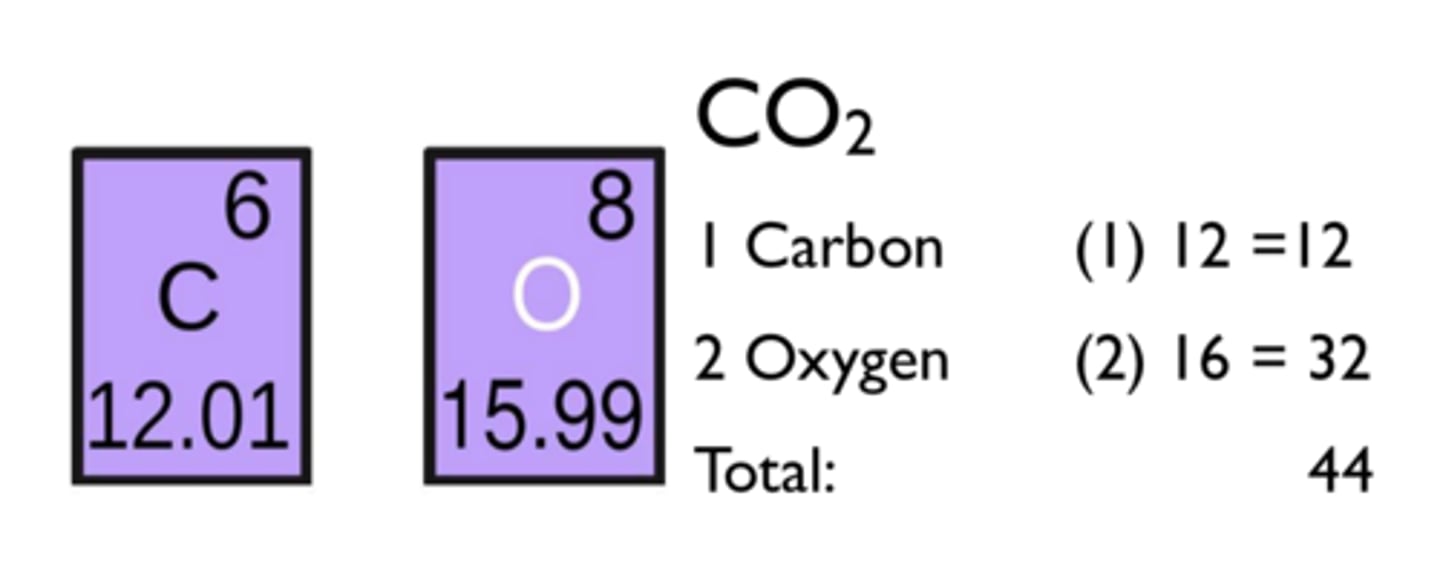
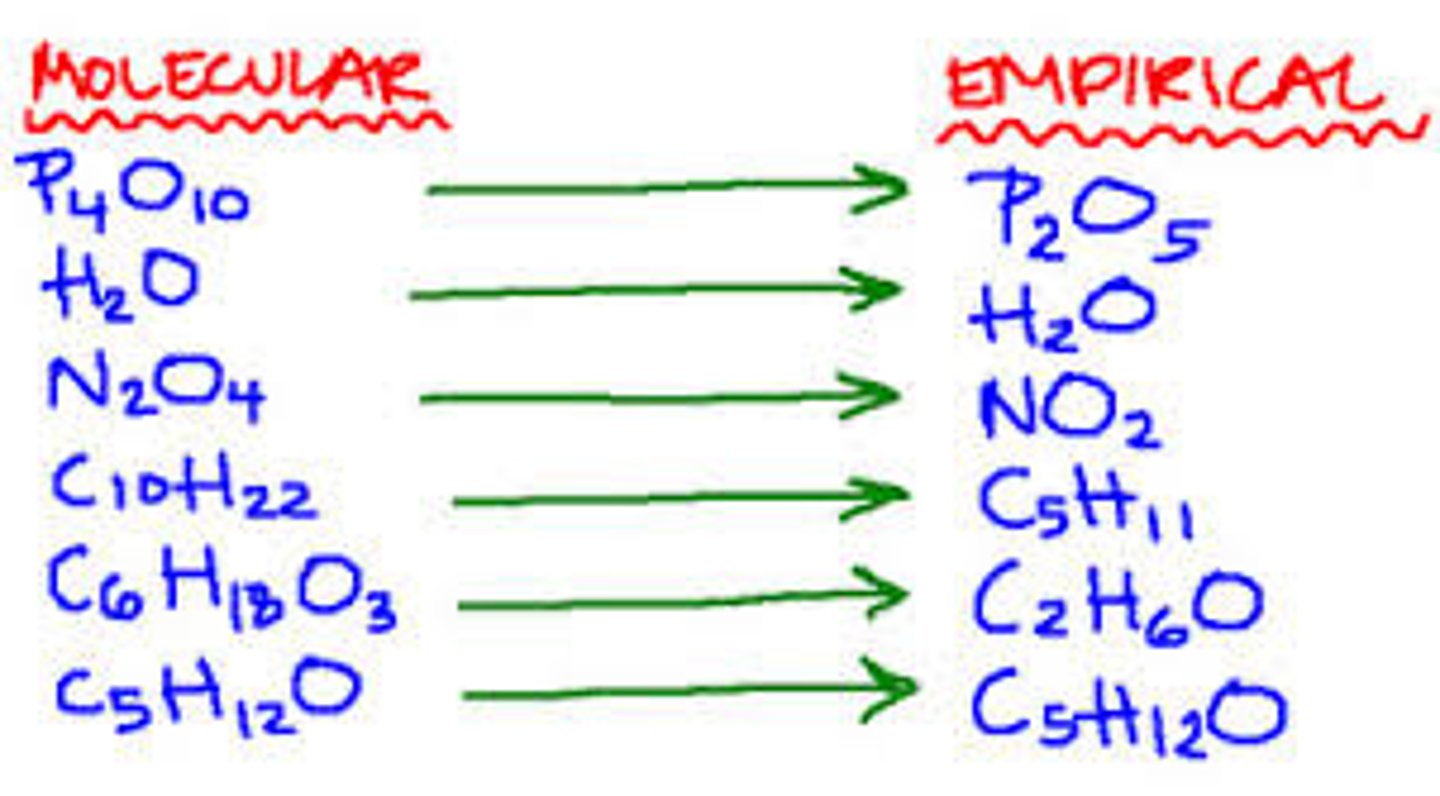
Molecular formula
A chemical formula that shows the number and kinds of atoms in a molecule
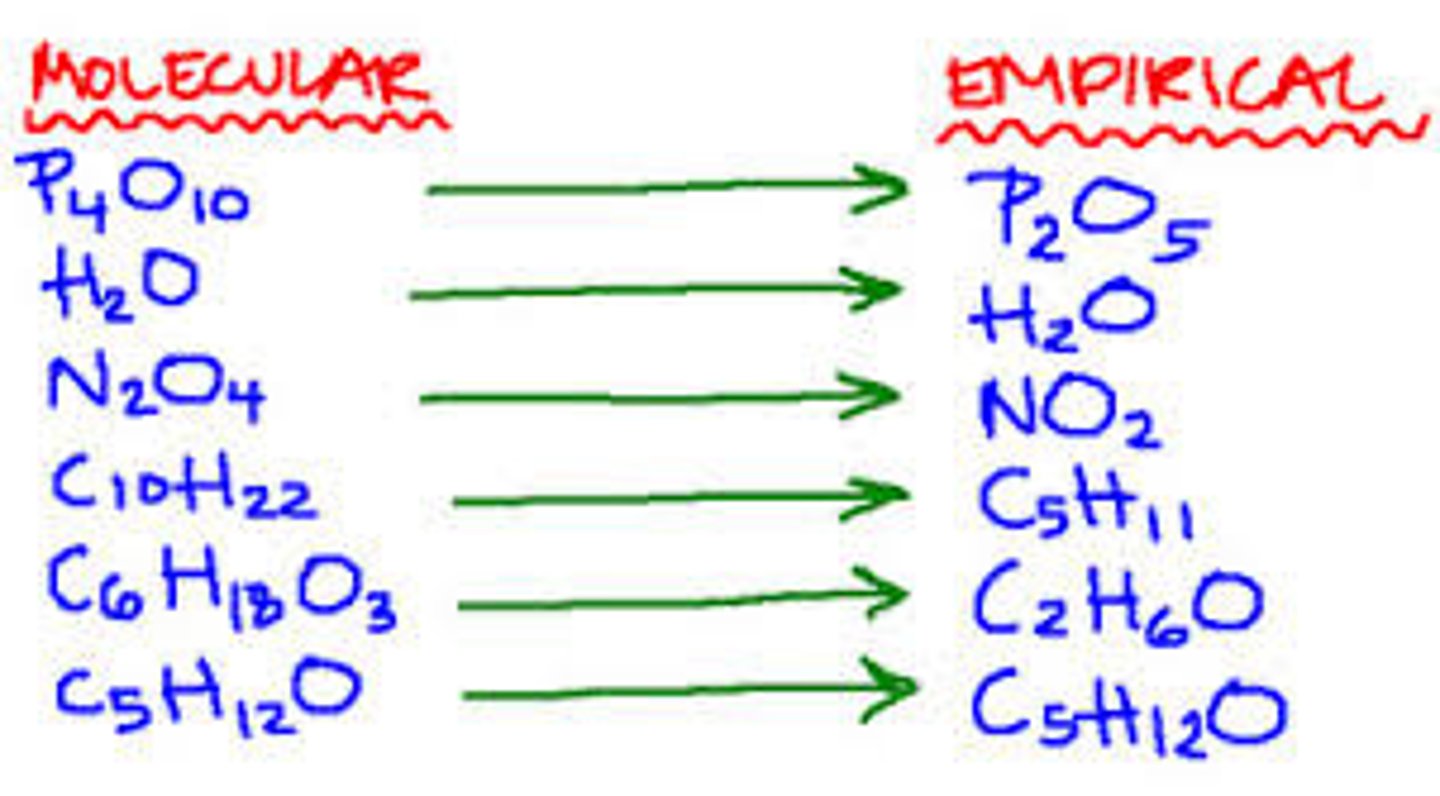
Empirical formula
The lowest whole-number ratio of elements in a compound
Relative formula mass
The sum of the relative atomic masses of the elements as given in the formula