Vaccines
1/110
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
111 Terms
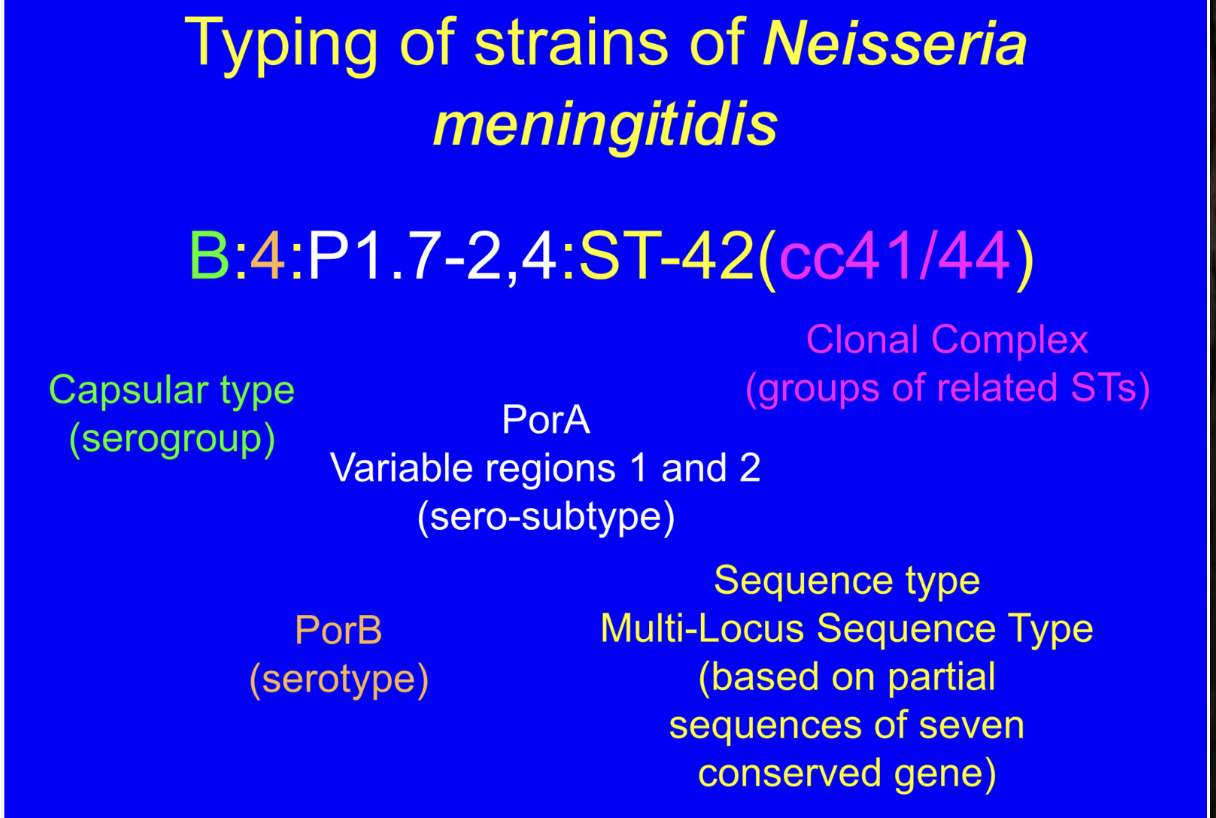
How to work out the types of strains of N. meningitides?
What are blebs?
vesicles containing LPS, glycerophspholipids, outer membrane proteins, periplasmic contents. They are specific to strains.
What are outer membrane vesicles prepared from?
strains subject to fermenter growth. The outer membrane is extracted with deoxycholate and absorbed to aluminium hydroxide
What is inside outer membrane vesicles?
glycerophospholipids, LPS, outer membrane proteins, reduced amount of LPS compared to blebs, PorA is the major immunogenic protein, many minor proteins
What does PorA do?
Elicits a strong immune response and antibodies made against it have bactericidal effects
What efficacy tests (and results) were done for OMV MenNZB vaccine?
variety of strains were tested and shown to generate a protective immune response, two doses for children 4+ and adults were 57-83% effective against homologous disease causing strain
Effectiveness of MeNZB vaccine.
no change in non-epidemic group B strains, rolled out in infant vaccination schedule until June 2008.
How do you overcome the problem of vaccines targeting specific strains?
find an antigen that is conserved and surface-expressed in all strains, check that the antigen has conserved surface-exposed epitopes, make sure that the conserved epitopes are highly immunogenicity, make sure the antigen elicits protective immune responses against a panel of strains representative of diversity in disease-causing organisms
What are recombinant vaccines?
Recombinant vaccines are vaccines created by inserting genes that code for specific pathogen antigens into a harmless virus or bacteria. This method allows for the production of immune responses without the use of live pathogens.
Why is the capsule of MenB not an ideal target for vaccine?
meningococcal serogroup B capsule is an alpha-2, 8-linked polysialic acid, structurally identical to neuronal cell adhesion molecule, poorly immunogenic, risk of inducing autoimmunity via cross-reactive antibodies
What is the method behind reverse vaccinology?
identifying genome sequence of a meningococcal strain, identify all the surface antigens, clone and express and raise antisera against surface antigens, test antisera for protective responses to homologous and heterologous strains
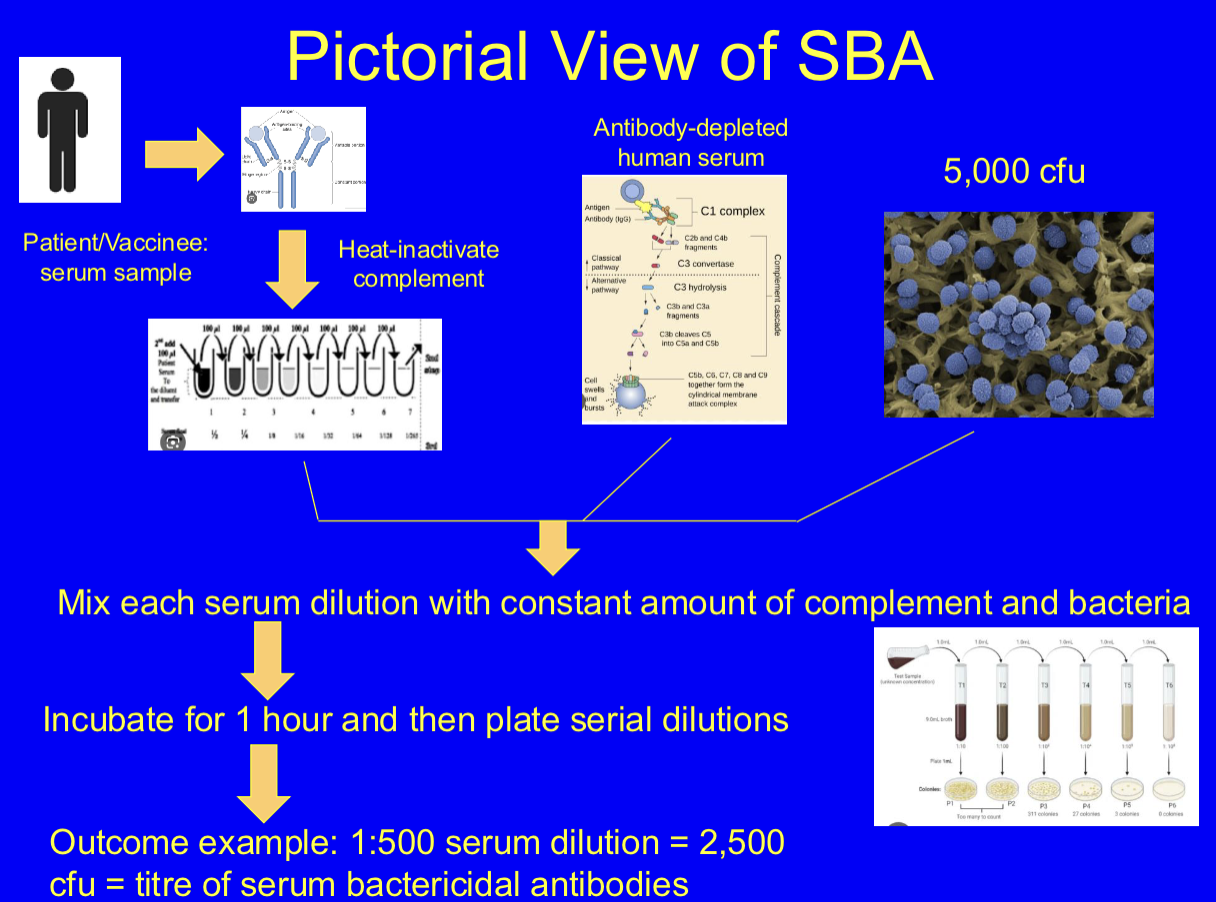
What is the serum bactericidal assay? (SBA)
gold-standard functional assay for assessing meningococcal protection. laboratory test that measures the ability of antibodies in a serum sample to kill bacteria. It's a key tool for evaluating the effectiveness of both vaccines and immune responses to infections, particularly in the context of complement-mediated killing.
Method for SBA.
meningococci grown on blood agar plates, inoculum of 5×10³ cry, mix with several dilutions of sera and a complement source, incubate for 1 hour at 37 degrees, plate dilutions and count and report titre as dilution in which there’s 50% survival
NZ combination vaccine.
The New Zealand OMV vaccine was shown to be highly effective, Combination of the recombinant antigens into this vaccine enhances the OMV responses, Combination of OMV and recombinant proteins utilised as generated responses to PorA (and possibly other minor antigens), improving vaccine coverage
What are the meninges?
The meninges are three protective membranes that cover the brain and spinal cord, consisting of the dura mater, arachnoid mater, and pia mater. They play a crucial role in protecting the central nervous system from infections and injury.
Where is the cerebrospinal fluid (CSF) located and what is its function?
Cerebrospinal fluid (CSF) is located in the space between the meninges, specifically in the subarachnoid space. Its primary function is to cushion the brain and spinal cord, providing a protective barrier and facilitating the exchange of nutrients and waste.
Why is the brain referred to as “immune privileged”?
The brain is considered "immune privileged" because it has limited access to immune cells and antibodies, allowing it to maintain a stable environment while reducing the risk of harmful inflammation.
What do glia limitans contains?
astrocytes with tight junctions formation in endothelial cells and prevent the transfer of particles, proteins and large molecules
What are astrocytes?
specialised macrophages in the brain that provide structural support, regulate blood flow, and maintain the blood-brain barrier. They can secrete pro- and anti-inflammatory factors.
Define the blood brain barrier (BBB).
The blood-brain barrier (BBB) is a selective permeability barrier formed by endothelial cells in the brain's blood vessels that protects the brain from potential toxins while allowing essential nutrients and molecules to pass through.
What are some of the functions of the BBB.
separates circulating blood from the fluid in the brain, prevents entry of large particles such as bacteria but permits diffusion of small molecules such as oxygen and carbon dioxide, allows for active transfer of other molecules such as glucose.
What are the BBB tight junctions composed of?
transmembrane proteins such as occluding, Claudius, junction adhesion molecules, anchor proteins
How is cerebrospinal fluid (CSF) made?
produced by filtration of blood through modified ependymal and epithelial cells in the choroid plexus (which exchanges 50-70% or around blood vessels)
What produces a large surface area in the choroid plexus which aids in the exchange of CSF?
choroid plexus contains microvilli which is folded into villi
Tight junctions in epithelial cells act as ______ barrier
blood-CSF barrier that regulates substance exchange
What is the choroid plexus?
A network of ependymal cells and blood vessels in the ventricles of the brain that produces cerebrospinal fluid (CSF).
How much CSF is produced daily? How much is present in the subarachnoid space?
500 ml/daily, 135-150 ml present in sub-arachnoid space
What are the contents in the CSF?
very little complement, proteins, no red blood cells, few white blood cells and IgG antibodies, very little transferon
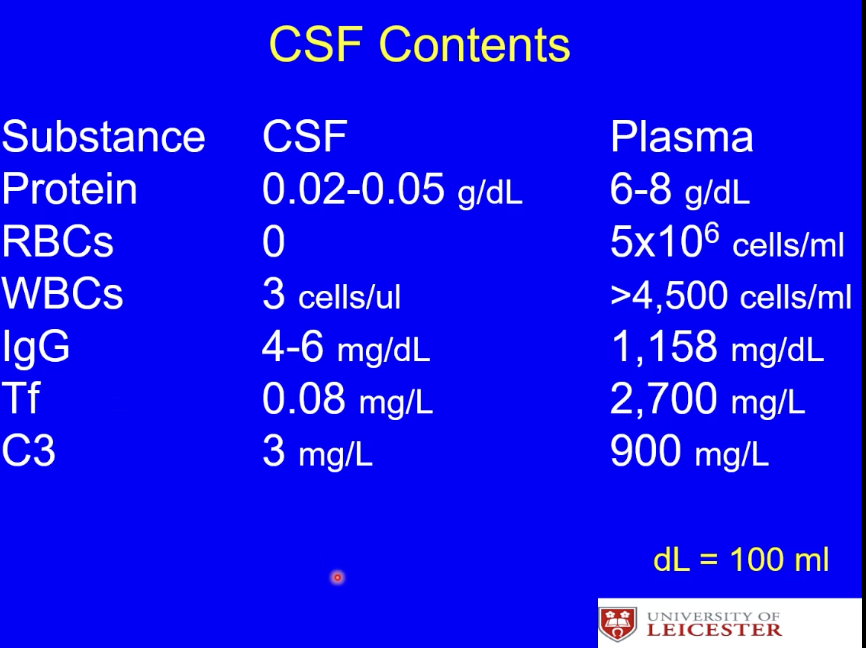
What does the contents of the CSF mean for its ‘immune’ abilities?
limited ability to respond to infections but also limited immune reactivity so less damage to the brain
What happens if bacteria such as N. meningitidis is able to enter the sub-arachnoid space?
bacterial replication and death in the cerebrospinal fluid which causes pro-inflammatory molecules such as LPS and peptidoglycan to be released. This stimulates the immune response as PAMPs induce cytokine release by meningeal macrophages and astrocytes
what is hib disease?
infection caused by Haemophilus influenza serogroup b
Who is affected by hib disease?
age dependent susceptibility, passive protection provided by maternal IgG for first 6 months
When do peak hib disease attacks occur?
at 6-7 months of age
High incidence of hib disease in ___ population.
unvaccinated
hib disease is uncommon after ___ years of age.
5
inverse correlation in hib disease between ____ and ___
between incidence and anticapsular/serum bactericidal antibodies
Why was the purified Haemophilus influenza serogroup b capsule used as a vaccine?
The purified Haemophilus influenzae serogroup b (Hib) capsule was used as a vaccine because it contains the key antigen (capsule is a polyribosyl-ribitol phosphate polysaccharide) that stimulates the immune system to produce antibodies against Hib bacteria, thus providing protection against invasive Hib disease.
What is the problem with using polysaccharides as vaccines?
Polysaccharide vaccines elicit a T-indepent immunity as there’s no antigen to present, resulting in a weaker immune memory.
What were the downsides of the purified capsule vaccine for hib disease?
The purified capsule vaccine for Hib disease had downsides such as a lack of long-lasting immunity and insufficient T-cell activation, leading to a weaker immune response compared to conjugate vaccines. Also showed a rapid decrease in antibody levels
Define conjugate vaccine.
A conjugate vaccine is a type of subunit vaccine that boosts the immune system's response to weak antigens by chemically linking them to a stronger carrier protein.This "conjugation" helps the immune system recognize and remember the weak antigen better, particularly in young children, who may have less developed immune systems
What was done to address the issues from the Hib disease capsular vaccine?
epidemiological studies
What did the epidemiological studies for the hib disease capsular vaccine include?
lab confirmed Hib as the causative agent of disease cases, stratification of the infections by age group and type of infection, denominator of the numbers of infants and children in the study population
What were the findings and results of the epidemiological studies for the hib disease capsular vaccine?
age range of deaths 2 months to 5 years old, 70% of infections were meningitis, meant that it should be introduced to children in the UK. Result was number of cases declined between 1990-2003.
Give an overview of N. meningitidis.
obligate commensal pathogen of humans, present in 10-15% of general population but can rise to 60% in enclosed populations, age association of disease with 50% of cases in under 5’s but significant peaks in late teens
What are the three types of meningococcal disease?
septicemia, meningitis, and a combination.
Define septicaemia.
Bacteria replicates in blood and releases LPS which induces endotoxic shock and involves leakage of fluid from the blood into tissues. One of the last steps of meningococcal disease. Severe and may require resuscitation or amputation.
What are some of the symptoms of septicaemia?
Fever, chills, rash, rapid breathing, and severe pain.
Death rate for septicaemia.
1 in 5.
Death rate for meningitis.
1 in 20
Which serogroup of meningococcal is most severe and a vaccine was developed?
serogroup C
What was the MenC-conjugate vaccine purified from and conjugated to?
purified meningococcal cultures from serogroup C and conjugated to tetanus toxoid
How does herd immunity occur?
due to natural infections in a significant proportion of the population providing protection to non-immune individuals
How does herd protection occur?
when vaccination in a significant proportion of the population provides protection to non-immune individuals. This is due to disruption in the transmission events as there’s a low probability of finding a non-immune host.
How did they test for herd immunity regarding the MenC vaccine?
took nasopharyngeal swabs from teenagers pre-vaccination to indicate the baseline level of MenC carriage in the population. As a control, the same locations are sampled 2 and 3 years later to examine if the levels of MenC carriage had changed whilst examining the carriage of other meningococcal capsular types as a control. The swabs are grown on selective media which selects for meningococci. The bacteria are isolated and tested for the presence of the capsule using antibodies which indicates the capsule is expressed and present on the bacterial surface and by PCR for capsule biosynthetic genes which indicates the capsular type.
Where are meningococci present?
nasopharyngeal tissues in the nose and back of the throat
Give examples of vaccines developed against bacterial meningitis.
hib disease capsular vaccine, MenC conjugate vaccine, MenA-conjugate vaccine
What were the benefits of the MenC vaccination for herd immunity?
induced a reduction in carriage of serogroup C strains, contributed to effectiveness of the vaccine and the reduction of serogroup C related disease, vaccine more cost-effective as no need to vaccinate other age-groups.
What does carriage mean?
'Carriage' means the presence of meningococcal bacteria in the upper respiratory tract without any signs or symptoms of infection.
Why was the MenA vaccine produced?
The MenA vaccine was produced to provide immunization against serogroup A meningococcal disease, which was responsible for significant morbidity and mortality in certain regions, particularly in the African meningitis belt.
What is the demographic or target group for the MenAfrVacc?
The target group for the MenAfrVacc includes children and adolescents in the African meningitis belt, particularly those aged between 1 and 29 years, to combat the high incidence of serogroup A meningococcal disease.
What kind of protection does MenAfrVacc give?
short-term protection against meningococcal disease
Why is the Men-C conjugate vaccine highly effective?
due to direct and indirect herd immunity protection
Define antigenicity.
The ability of a substance to provoke an immune response by binding to an antibody or T-cell receptor.
Define adjuvants.
Adjuvants are substances that enhance, prolong and accelerate induction of the immune response to a vaccine antigen. They reduce the amount of antigen required in the vaccine and provide a general stimulus to the immune system.
How do adjuvants work?
retain and concentrate antigens in location of lympocytes (‘Depot effect’) or deliver antigens to APCs. Induce cytokine production and regulate lymphocyte activity.
How can adjuvants direct the immune response?
Adjuvants can direct the immune response by binding to a pattern recognition receptor (PRR). Different cytokines are produced depending on which receptor is ligated. Depending on which adjuvant used, you can lean the response to a bias to a Th1 or Th2 direction.
What adjuvants are used for vaccines?
natural, synthetic or endogenous. Main used are aluminium gels.
How do vaccines work?
elicit a high affinity, long-lasting, specific immune response
What is the active component in different vaccines?
can include toxoid (inactivated toxin), toxin sub-unit, polysaccharide (based on a capsule and must be linked to carrier protein), live bacteria, killed bacteria
What does the current UK infant vaccine schedule contain?
The current UK infant vaccine schedule contains immunizations against diseases such as DTaP/IPV/Hib, pneumococcal disease and meningitis B, along with MMR.
How do vaccines work?
induced immunity, immune memory, location of immunity, adjuvants
Describe the immunology of vaccinology.
immature B-cells bind to and recognise antigen and produce IgM. They then produce a germinal centre and start to proliferate and secrete antibodies from the lymph nodes. Follicular dendritic cells then present the antigen to B-cells and select for high affinity. This then leads to differentiation and affinity maturation due to somatic hypermutation and VDJ recombination, resulting in memory B-cells and long-lasting immunity.
What do naive B cells produce when bound to an antigen that triggers proliferation of antigen-specific cells due to support of CD4+ T cells?
primarily long-lived plasma B cells but also memory B cells
What do naive T cells produce when bound to an antigen that triggers proliferation of antigen-specific cells?
Central memory T cells (Tcm) and primarily effector memory T cells (Tem) which circulate in the blood or remain in the tissue
Define opsonisation.
The process by which pathogens are marked for destruction by immune cells through the binding of antibodies or complement proteins, enhancing their recognition and uptake.
What type of response do mature plasma B-cells produce?
immediate antibody response to pathogens.
What type of response do memory B-cells produce?
delayed antibody response upon re-exposure to the same antigen.
What kind of antibodies can be generated from mature plasma B cells?
neutralising, opsonising, and activating complement antibodies.
How do neutralising antibodies work?
Neutralising antibodies bind to specific antigens on pathogens, blocking their ability to infect cells and preventing disease progression.
How fast is the naive cell (initial) response?
5-10 days
How fast is the memory B cell (vaccine) response?
2-5 days
What is the difference in the antibody type affinity between the primary and secondary response?
The primary response produces low affinity IgM antibodies, while the secondary response generates high IgG and IgA affinity antibodies due to affinity maturation.
How many subclasses does IgG have?
IgG has four subclasses: IgG1, IgG2, IgG3, and IgG4.
What are the targets of IgG1 and IgG3?
target proteins
What does IgG2 target?
targets polysaccharides
What type of mechanism does IgA have?
blocking mechanism (so not killing or opsonic like IgG)
Immunoglobulins are produced by?
the spleen, regional lymph nodes, submucosal lymphoid tissue such as the MALT, NALT and Peyer's patches.
How many types of IgA are there?
monomeric found in the blood or serum (IgA1) and dimeric/polymeric found in secretions (IgA2 in lower GI tract)
Why is IgG known as the functional antibody?
Because it can opsonize pathogens, activate complement, and mediate antibody-dependent cellular cytotoxicity (ADCC), providing strong immune defense.
What are some key characteristics of vaccines?
must be efficacious, produce long-lasting protection, must be broad-spectrum, must be safe, must be stable and easily administered, must be easy to deliver, and must induce a strong immune response that could aid in herd immunity.
What are some key characteristics when thinking of developing a vaccine program? 5!
age or target group, cost-benefit ratio (number of cases, hospitalisation costs, unit cost of vaccine, generates herd immunity), effect on commensals (does it serve a risk to natural immunity), potential for bacteria to escape vaccine, impact on other vaccines
What are the stages in vaccine development?
target validation (identifying conserved surface antigen), testing for immunogenicity and generation of a protective immune response that produces antibodies, testing for coverage of all pathogenic strain of that organism, safety testing to understand function, thinking on how you’ll present the antigen, preparation of vaccine batch
Who invented first Whooping Cough Vaccine?
Pearl Kendrick and Grace Eldering developed the first whooping cough vaccine in the 1930s, significantly reducing its incidence.
What causes whooping cough?
Bordetella pertussis
What are some of the characteristics of Bordetella pertussis?
small, gram negative, aerobic coccobacillus, fermentative, thrives in blood-rich areas
What are some of the characteristics of whooping cough?
highly infectious, colonises upper respiratory and can cause pneumonia if it enters lower respiratory tract, complications can lead to seizures and pneumonia
What are some of the symptoms of whooping cough?
cold-like symptoms, dry cough which can worsen to prolonged coughing bouts
Describe the background of the Pertussis Field Trial.
Trialed vaccine over three years in 5,815 children, control group was of a similar size, age and districts as immunised group, vaccinated group was “all children of an acceptable age and history who presented themselves for vaccination”.
What was a necessary step that Kendrick and Eldering needed to do prior the the trial starting?
Assess the levels of disease in the population to determine baseline incidence rate (how long the disease lasted, when the infected individuals were producing infectious organisms)
How did Eldering and Kendrick assess the baseline incidence rate for B. pertussis?
developed a new growth media which allowed for faster and more abundant growth of b. pertussis colonies which improved diagnostics. This media was then offered as a cough plate to doctors.