Module 2 - Atoms and Reactions
0.0(0)
Card Sorting
1/86
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
87 Terms
1
New cards
\[2.1.1\] What is an **ISOTOPE**?
Atoms of the same element with different numbers of neutrons and different masses.
2
New cards
\[2.1.1\] What is meant by the term '**RELATIVE** **ISOTOPIC** **MASS**'?
The mass compared with 1/12th the mass of carbon-12.
3
New cards
\[2.1.1\] What is meant by the term '**RELATIVE** **ATOMIC** **MASS**'?
The weighted mean mass compared with 1/12th the mass of carbon-12.
4
New cards
\[2.1.1\] What is **MASS SPECTROMETRY**?
Mass Spectrometers allow the percentage abundances of the isotopes in a sample of an element to be found.
They work to the principles of:
1) A sample is placed in the mass spectrometer.
2) Sample is vaporised and then ionised to form cations.
3) Ions are accelerated. Heavier ions move more slowly and are more difficult to deflect than lighter ions, so the ions of each isotope are separated.
4) The ions are detected on a mass spectrum as a mass to charge ratio (m/z). Each ion reaching the detector adds to the signal, so the greater the abundance, the greater the signal.
For ions with one positive charge, this ratio is equivalent to the relative isotopic mass, which is recorded on the x-axis.
They work to the principles of:
1) A sample is placed in the mass spectrometer.
2) Sample is vaporised and then ionised to form cations.
3) Ions are accelerated. Heavier ions move more slowly and are more difficult to deflect than lighter ions, so the ions of each isotope are separated.
4) The ions are detected on a mass spectrum as a mass to charge ratio (m/z). Each ion reaching the detector adds to the signal, so the greater the abundance, the greater the signal.
For ions with one positive charge, this ratio is equivalent to the relative isotopic mass, which is recorded on the x-axis.
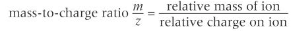
5
New cards
[2.1.2] What are the main ions?
NO₃⁻, CO₃²⁻, SO₄²⁻, OH⁻, NH₄⁺, Zn²⁺, Ag⁺
6
New cards
\[2.1.3\] What is a **MOLE**?
A mole is the unit for amount of substance, symbol 'mol'.
7
New cards
[2.1.3] What is the equation linking Moles, Mass and Molar Mass?
Moles = Mass / Molar Mass

8
New cards
[2.1.3] What is the equation linking Moles, Amount of Substance and Avogadro's Constant?
Moles = Amount of Substance / Avogadro's Constant
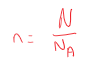
9
New cards
[2.1.3] What is the equation linking Moles, Concentration and Volume?
Moles = Concentration x Volume
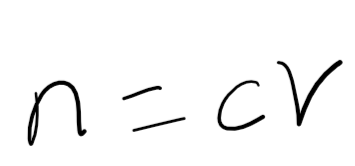
10
New cards
\[2.1.3\] What is **molar concentration**?
Number of ***moles of solute per dm³ of solvent***. It has the units **moldm⁻³**.
11
New cards
\[2.1.3\] What is **mass concentration**?
Number of ***grams of solute per dm³ of solvent***. It has the units **gdm⁻³**.
12
New cards
\[2.1.3\] How do we convert between mass concentration and molar concentration?
Mass Concentration / Molar Mass = Molar Concentration
\
Molar Concentration x Molar Mass = Mass Concentration
\
Molar Concentration x Molar Mass = Mass Concentration
13
New cards
\[2.1.3\] What is the **molar gas volume**?
The **volume per mole of gas molecule at a stated temperature and pressure**.
14
New cards
\[2.1.3\] What is **RTP**?
**20°C** and **101kPa** (**1 atm**).
15
New cards
\[2.1.3\] What is the volume of 1 mole of gas molecules at RTP?
24\.0dm³
16
New cards
\[2.1.3\] Which equation links moles, volume and molar gas volume?
n (moles) = V (volume) / Vₘ (molar gas volume)
17
New cards
\[2.1.3\] What are the assumptions for the molecules within an **ideal gas**?
* **Random Motion**
\
* **Elastic Collisions**
\
* **Negligible** Size
\
* **No Intermolecular Forces**
\
* **Elastic Collisions**
\
* **Negligible** Size
\
* **No Intermolecular Forces**
18
New cards
\[2.1.3\] What is the equation that links pressure, volume, moles, temperature and the ideal gas constant?
Pressure (P) (Pa) x Volume (V) (m³) = Moles (n) (mol) x Ideal Gas Constant (R) (8.31Jmol⁻¹K⁻¹) x Temperature (T) (K)
19
New cards
\[2.1.3\] What is **water of crystallisation**?
Water molecules that are bonded into a **crystalline structure**.
20
New cards
\[2.1.3\] What is meant by the term **hydrated**?
When the crystalline structure contains water.
21
New cards
\[2.1.3\] What is meant by the term **anhydrous**?
When the crystalline structure does not contain water.
22
New cards
\[2.1.3\] What is the equation for **percentage yield**?
% Yield = (Actual Yield / Theoretical Yield) x 100
23
New cards
\[2.1.3\] Why do most reactions not result in a 100% yield?
* Side Reactions
* Most reactions are not completed, most have some form of **reversibility**.
* Systematic Errors.
* Most reactions are not completed, most have some form of **reversibility**.
* Systematic Errors.
24
New cards
\[2.1.3\] What is the equation for **atom economy**?
Atom Economy = (Mᵣ of Desired Product / Mᵣ of All Products) x 100
25
New cards
\[2.1.4\] What is an **acid**?
A substance that **donates a proton**. They contain **hydrogen**.
26
New cards
\[2.1.4\] What occurs when acids are dissolved in water?
The acid releases **hydrogen ions** into the solution, e.g. for HCl:
\
HCl(g) + aq (excess of water) → H⁺ + Cl⁻
\
HCl(g) + aq (excess of water) → H⁺ + Cl⁻
27
New cards
\[2.1.4\] What is a **strong acid**?
A substance which **releases all of its hydrogen atoms** into solution as H⁺ ions and completely **dissociates** in aqueous solution.
\
HA → H⁺ + A⁻
\
HA → H⁺ + A⁻
28
New cards
\[2.1.4\] What are the main strong acids?
Sulfuric Acid, Hydrochloric Acid, Nitric Acid, Perchloric Acid, Chloric Acid, Hydrobromic Acid and Hydroiodic Acid
29
New cards
\[2.1.4\] What is a **weak acid**?
A substance that only **releases a small proportion of its hydrogen atoms** into solution as H⁺ ions. It only **partially dissociates** in aqueous solution.
\
HA ⇌ H⁺ + A⁻
\
HA ⇌ H⁺ + A⁻
30
New cards
\[2.1.4\] What is a **base**?
A substance which accepts **protons** in a reaction.
31
New cards
\[2.1.4\] How are **salts** formed?
A **base** **NEUTRALISES** an **acid**.
32
New cards
\[2.1.4\] What is a **salt?**
When the positive H⁺ ions are **replaced** with a different cation.
33
New cards
\[2.1.4\] Which substances are examples of bases?
* Metal Oxides
* Metal Hydroxides
* Metal Carbonates
* Ammonia
* Metal Hydroxides
* Metal Carbonates
* Ammonia
34
New cards
\[2.1.4\] What is an **alkali**?
A base which **dissolves in water**, releasing **hydroxide ions** into the solution.
\
e.g. NaOH(s) + aq → Na⁺(aq) + OH⁻
\
e.g. NaOH(s) + aq → Na⁺(aq) + OH⁻
35
New cards
\[2.1.4\] What are the main **neutralisation** reactions?
* Acid + Alkali → Salt + Water
\
* Acid + Metal Oxide → Salt + Water
\
* Acid + Metal Hydroxide → Salt + Water
\
* Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
\
* Acid + Metal → Salt + Hydrogen Gas
\
* Acid + Metal Oxide → Salt + Water
\
* Acid + Metal Hydroxide → Salt + Water
\
* Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
\
* Acid + Metal → Salt + Hydrogen Gas
36
New cards
\[2.1.5\] What are **oxidation numbers**?
The **number of electrons** that an atom uses to **bond with atoms of another element**.
37
New cards
\[2.1.5\] What is the first rule of oxidation states?
**UNCOMBINED ELEMENT** - Oxidation Number = **0**, e.g. Na or O₂.
→ All electrons are being shared perfectly.
→ All electrons are being shared perfectly.
38
New cards
\[2.1.5\] What is the second rule of oxidation states?
**COMBINED OXYGEN** - Oxidation Number = **-2**, e.g. H₂O or CaO.
→ Exceptions to this rule include **H₂O₂** or if ***oxygen is bonded to fluorine***.
→ Exceptions to this rule include **H₂O₂** or if ***oxygen is bonded to fluorine***.
39
New cards
\[2.1.5\] What is the third rule of oxidation states?
**COMBINED HYDROGEN** - Oxidation Number = **+1**, e.g. H₂S.
→ Exceptions to this rule include **metal hydrides** (NaH) whereby Hydrogen has an oxidation number of **-1**.
→ Exceptions to this rule include **metal hydrides** (NaH) whereby Hydrogen has an oxidation number of **-1**.
40
New cards
\[2.1.5\] What is the fourth rule of oxidation states?
**SIMPLE IONS** - Oxidation Number = **Charge of Ion**, e.g. Na⁺ (+1)
41
New cards
\[2.1.5\] What is the fifth rule of oxidation states?
**COMBINED FLUORINE** - Oxidation Number = **-1**, e.g. NaF, NaF₂.
42
New cards
\[2.1.5\] What is the sixth rule of oxidation states?
**THE SUM OF ALL THE OXIDATION NUMBERS MUST EQUAL THE OVERALL CHARGE OF THE COMPOUND IN QUESTION**.
43
New cards
\[2.1.5\] What are **oxyanions**?
Formed when **oxygen** reacts with other elements to form **anions**.
\
e.g. Nitrate (III) and Nitrate (V) are both oxyanions.
\
e.g. Nitrate (III) and Nitrate (V) are both oxyanions.
44
New cards
\[2.1.5\] What is **oxidation**?
* The **loss of electrons**.
\
* The **increase in oxidation number**.
\
* The **addition of oxygen**.
\
* The **increase in oxidation number**.
\
* The **addition of oxygen**.
45
New cards
\[2.1.5\] What is an **oxidising agent**?
* Is normally a **non-metal** or **cation**.
\
* Causes **oxidation** to take place.
\
* **Gains electrons** from other atoms or ions so is itself **reduced**.
\
* Causes **oxidation** to take place.
\
* **Gains electrons** from other atoms or ions so is itself **reduced**.
46
New cards
\[2.1.5\] What is **reduction**?
* The **gain of electrons**.
\
* The **decrease in oxidation number**.
\
* The **removal of oxygen**.
\
* The **decrease in oxidation number**.
\
* The **removal of oxygen**.
47
New cards
\[2.1.5\] What is a **reducing agent**?
* Usually a **metal** or **anion**.
\
* **Donates electrons** to another species.
\
* Is itself **oxidised**.
\
* **Donates electrons** to another species.
\
* Is itself **oxidised**.
48
New cards
\[2.1.5\] What is **disproportionation**?
The same element has been both **reduced** __**AND**__ **oxidised** in the same reaction.
49
New cards
\[2.1.5\] How do we work out **half-equations**?
1. Balance the equation other than hydrogen and oxygen.
2. Balance *oxygen* by adding **water**.
3. Balance *hydrogen* by adding **hydrogen ions**.
4. Balance *charge* by adding **electrons**.
1. Add **state symbols**.
50
New cards
\[2.2.1\] What are electron energy levels made up of?
Energy levels are made up of **subshells** which are further made up of **orbitals**.
51
New cards
\[2.2.1\] What is an **orbital**?
A **region around the nucleus** that can hold up to **2 electrons** with **opposite spin**.
52
New cards
\[2.2.1\] What is the shape of an **S orbital**?
**Spherical**.
53
New cards
\[2.2.1\] What is the shape of a **P orbital**?
‘Dumbbell’ shaped. They exist at **right-angles** to eachother.
54
New cards
\[2.2.1\] What is the **aufbau principle**?
Electrons fill **lower energy orbitals** before **higher energy** orbitals.
55
New cards
\[2.2.1\] What is **hund’s rule of maximum multiplicity**?
Every orbital in a subshell is **singly occupied** with one electron before any orbital is **doubly occupied**.
56
New cards
\[2.2.1\] Why does the **4s** orbital fill before the **3d** orbital?
When the 4s orbital has *no electrons*, it has a **LOWER energy** than the 3d orbital but when it *has electrons*, it has a **HIGHER energy**.
57
New cards
\[2.2.2\] What is **ionic bonding**?
The **strong electrostatic force of attraction** between **oppositely charged ions**.
58
New cards
\[2.2.2\] How can **melting and boiling points** of ionic compounds be explained?
At room temperature, there is **insufficient energy** to overcome the **strong electrostatic forces within the giant ionic lattice structures**. This means that high temperatures are required to provide the energy needed to overcome these forces.
59
New cards
\[2.2.2\] How can the **solubility** of ionic compounds be explained?
* Many ionic compounds **dissolve in polar solvents**. Polar water molecules ***break down the lattice*** and **surround each ion** in solution.
\
* In compounds made of ions with **large charges**, the **ionic attraction may be too strong** to overcome. This means that the compound is not very soluble.
\
* In compounds made of ions with **large charges**, the **ionic attraction may be too strong** to overcome. This means that the compound is not very soluble.
60
New cards
\[2.2.2\] How can the **electrical conductivity** of ionic compounds be explained?
For an ionic compound to conduct electricity, it must be **molten** or **aqueous**. This is because the ionic lattice breaks down and the ions become **free to move** as **mobile charge carriers**.
61
New cards
\[2.2.2\] What is **covalent bonding**?
The **strong electrostatic attraction** between a **shared pair of electrons** and the **nuclei of the bonded atoms**.
62
New cards
\[2.2.2\] Why does **sulfur** expand its **octet**?
The **d-subshell** has space for **electrons**.
63
New cards
\[2.2.2\] What is **dative**/**coordinate covalent bonding**?
A **shared pair of electrons** which has been provided by only **one** of the bonding atoms, e.g. in NH₄⁺.
64
New cards
\[2.2.2\] What is **average bond enthalpy**?
A measure of **covalent bond strength**. The larger the **bond enthalpy**, the **stronger** the covalent bond.
65
New cards
\[2.2.2\] How much does a **lone pair** reduce a bond angle by?
**2.5°.**
66
New cards
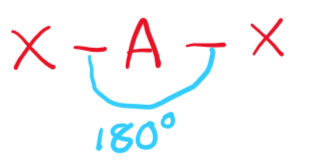
\[2.2.2\] What is the molecular shape with 2 bonding pairs and no lone pairs?
A **linear** shape, e.g. CO₂ or HCN.
\
It has a bond angle of **180°**.
\
It has a bond angle of **180°**.
67
New cards
\[2.2.2\] What is the molecular shape with 2 bonding pairs and 1 lone pair?
A **non-linear** shape, e.g. SO₂.
\
It has a bond angle of **115° - 120°**.
\
It has a bond angle of **115° - 120°**.
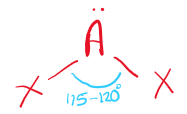
68
New cards
\[2.2.2\] What is the molecular shape with 2 bonding pairs and 2 lone pairs?
A **non-linear** shape, e.g. H₂O.
\
It has a bond angle of **104.5°**.
\
It has a bond angle of **104.5°**.
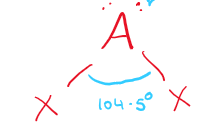
69
New cards
\[2.2.2\] What is the molecular shape with 3 bonding pairs and no lone pairs?
A **trigonal planar** shape, e.g. **Aldehydes**.
\
It has a bond angle of **120°**.
\
It has a bond angle of **120°**.
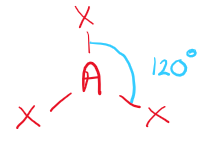
70
New cards
\[2.2.2\] What is the molecular shape with 3 bonding pairs and 1 lone pair?
A **trigonal pyramidal** shape, e.g. NH₃.
\
It has a bond angle of **107°**.
\
It has a bond angle of **107°**.

71
New cards
\[2.2.2\] What is the molecular shape with 4 bonding pairs and no lone pairs?
A **tetrahedral shape**, e.g. NH₄⁺.
\
It has a bond angle of **109.5°**.
\
It has a bond angle of **109.5°**.
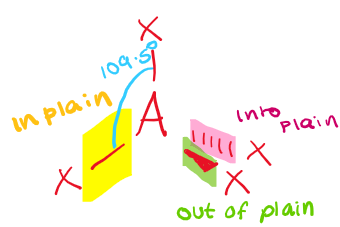
72
New cards
\[2.2.2\] What is the molecular shape with 4 bonding pairs and 2 lone pairs?
A **square planar** **shape**.
\
It has a bond angle of **90°**.
\
It has a bond angle of **90°**.

73
New cards
\[2.2.2\] What is the molecular shape with 6 bonding pairs and no lone pairs?
An **octahedral shape**.
\
It has a bond angle of **90°**.
\
It has a bond angle of **90°**.
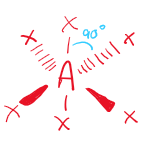
74
New cards
\[2.2.2\] What is the definition of **electronegativity**?
The ability of an atom to **attract a pair of electrons** within a **covalent bond**.
75
New cards
\[2.2.2\] How do we work out whether molecules are likely to be non-polar?
* **Symmetry**
\
* Dipoles **cancel out**.
\
* **No overall dipole**.
\
* Dipoles **cancel out**.
\
* **No overall dipole**.
76
New cards
\[2.2.2\] How does the **Pauling Scale** compare the electronegativity of the atoms of different elements?
* A large Pauling value indicates that atoms of the element are very electronegative.
\
* Electronegativity increases up the periodic table so Fluorine is the most electronegative with a value of **4.0**.
\
* Electronegativity increases up the periodic table so Fluorine is the most electronegative with a value of **4.0**.
77
New cards
\[2.2.2\] What are **London forces?**
**Weak intermolecular forces** that exist between __ALL MOLECULES____**.**__
\
They act between induced dipoles in different molecules.
\
They act between induced dipoles in different molecules.
78
New cards
\[2.2.2\] What causes **induced dipoles**?
1. The movement of **electrons** produces a **temporary dipole**.
2. At any instant, an **instantaneous dipole** will exist but its position is constantly changing.
3. The **instantaneous dipole** induces a dipole on a neighbouring molecule.
4. This induced dipole induces further dipoles which then attract.
79
New cards
\[2.2.2\] What happens to **induced dipole-dipole interactions** as the number of electrons present increases?
* The larger the instantaneous and induced dipoles.
* The greater the induced dipole-dipole interactions.
* The stronger the attraction between molecules.
* The greater the induced dipole-dipole interactions.
* The stronger the attraction between molecules.
80
New cards
\[2.2.2\] What are **permanent dipole-dipole interactions**?
Forces between the **permanent dipoles** in different **polar** molecules.
81
New cards
\[2.2.2\] Why is a **low melting and boiling point** a property of simple molecular substances?
In simple molecular lattices, the **weak intermolecular forces** can be broken by very small amounts of energy.
82
New cards
\[2.2.2\] Why is **solubility** a property of non-polar simple molecular substances?
When a simple molecular compound is added to a non-polar solvent, such as hexane, **intermolecular forces** form between the molecules and the solvent.
* The interactions **weaken** the intermolecular forces in the lattice and they break when the compound **dissolves**.
*Therefore, non-polar simple molecules tend to be* ***soluble*** *in non-polar solvents.*
\
* When a simple molecular substance is added to a **polar** solvent, there are few interactions between the lattice and the solvent.
* The intermolecular bonding within the solvent is **too strong** to be broken.
*Therefore non-polar simple molecular substances are usually* ***insoluble*** *in polar solvents.*
* The interactions **weaken** the intermolecular forces in the lattice and they break when the compound **dissolves**.
*Therefore, non-polar simple molecules tend to be* ***soluble*** *in non-polar solvents.*
\
* When a simple molecular substance is added to a **polar** solvent, there are few interactions between the lattice and the solvent.
* The intermolecular bonding within the solvent is **too strong** to be broken.
*Therefore non-polar simple molecular substances are usually* ***insoluble*** *in polar solvents.*
83
New cards
\[2.2.2\] Why is **solubility** a property of polar simple molecular substances?
Polar covalent substances can dissolve in polar solvents because the **polar solute molecules** and the **polar solvent molecules** can **attract** each-other in a process similar to the dissolving of an ionic compound.
The solubility depends on the strength of the **dipole.**
The solubility depends on the strength of the **dipole.**
84
New cards
\[2.2.2\] Why is **electrical conductivity** not a property of simple molecular substances?
* There are **no mobile charged particles**, meaning there is **nothing to complete an electrical circuit** so simple molecules cannot conduct electricity.
85
New cards
\[2.2.2\] What is **hydrogen bonding**?
A type of **permanent dipole-dipole interaction** found between molecules containing:
\
* An **electronegative atom** with a **lone pair** of electrons.
* A **hydrogen atom** attached to an **electronegative atom**.
\
They are the strongest type of intermolecular attraction.
\
* An **electronegative atom** with a **lone pair** of electrons.
* A **hydrogen atom** attached to an **electronegative atom**.
\
They are the strongest type of intermolecular attraction.
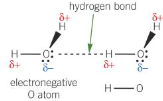
86
New cards
\[2.2.2\] Why is **ice** more dense than water?
* Hydrogen bonds hold water molecules apart in an **open lattice**.
* The water molecules in ice are further apart than in water.
\
Each water molecule forms **4 hydrogen bonds** which extend outwards, holding the water molecules slightly apart in an **open tetrahedral lattice full of holes** which decrease the density of water on freezing.
* The water molecules in ice are further apart than in water.
\
Each water molecule forms **4 hydrogen bonds** which extend outwards, holding the water molecules slightly apart in an **open tetrahedral lattice full of holes** which decrease the density of water on freezing.
87
New cards
\[2.2.2\] Why does water have a **relatively high melting and boiling point?**
Water contains **London forces** between its molecules.
\
* Hydrogen bonds are **extra** forces, over and above the London forces.
* A **high quantity of energy** is needed to break these hydrogen bonds so it has a higher melting and boiling point than would be expected just from London forces.
\
* Hydrogen bonds are **extra** forces, over and above the London forces.
* A **high quantity of energy** is needed to break these hydrogen bonds so it has a higher melting and boiling point than would be expected just from London forces.