2 - Spectroscopy and Lab Techniques
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
48 Terms
chromatography:
have 2 phases (stationary and mobile) and you are running 2 mixtures AGAINST each other
stationary phase:
substance that SUPPORTS that mixture and allows compounds to be retained
mobile phase:
fluid that carries mixture of compounds to be SEPARATED
characteristics of chromatography:
- passing the mobile phase along the stationary phase allows compounds to be distributed around them
- greater affinity/attraction to stationary phase means that the LONGER that compound is retained (SMALLER Rf value)
methods of collection chromatography:
size exclusion, column, HPLC, ion-exchange, affinity
methods of analysis chromatography:
TLC, gas chromatography
size exclusion chromatography:
separate based on MOLECULAR SIZE (big vs. small)
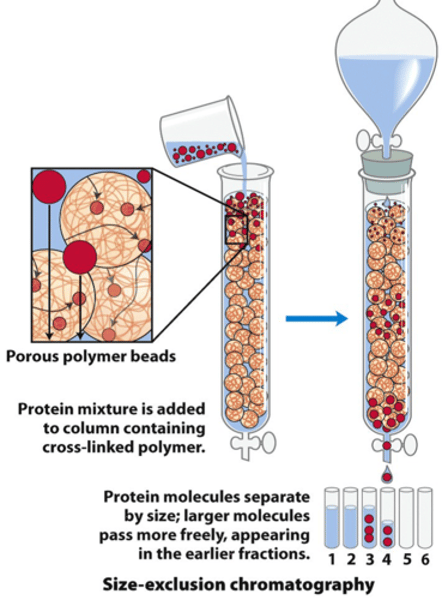
Stationary & mobile phases of size exclusion chromatography:
column is packed w/ INERT, porous beads (stationary phase)
- stationary phase: smaller compounds elute LAST (b/c they get STUCK so travel longer and less direct path)
- mobile phase: larger compounds elute FIRST (b/c they're exempt from being stuck in bead)
thin layer chromatography (TLC):
used to separate SMALL amounts of high molecular weight compounds (analysis) via POLARITY
Stationary and mobile phases of TLC:
- stationary phase: plate is covered w/ SILICA GEL (polar) therefore polar compounds travel LESS
- mobile phase: shallow solvent bath within sealable development chamber that makes nonpolar compounds travel MORE
why do polar compounds travel less in TLC?
they have stronger interactions w/ silica gel (H-bonded)
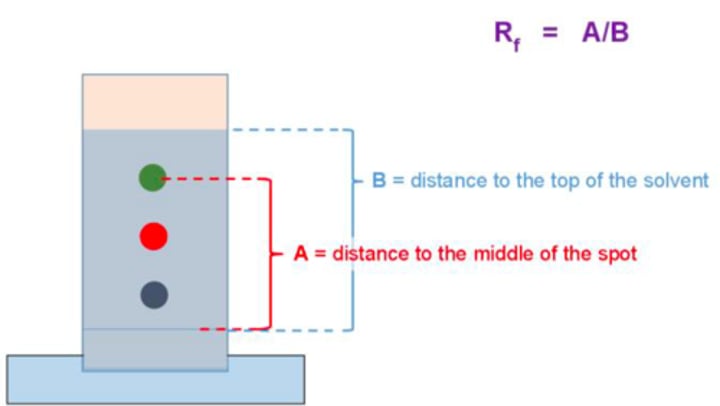
Rf value:
tells you how far the compound has traveled until solvent front (Rf = spot / solvent front)
- Nonpolar compounds have HIGHER Rf values
- Polar compounds have LOWER Rf values
Column chromatography:
to collect so therefore use LARGE amounts of a compound, based on POLARITY
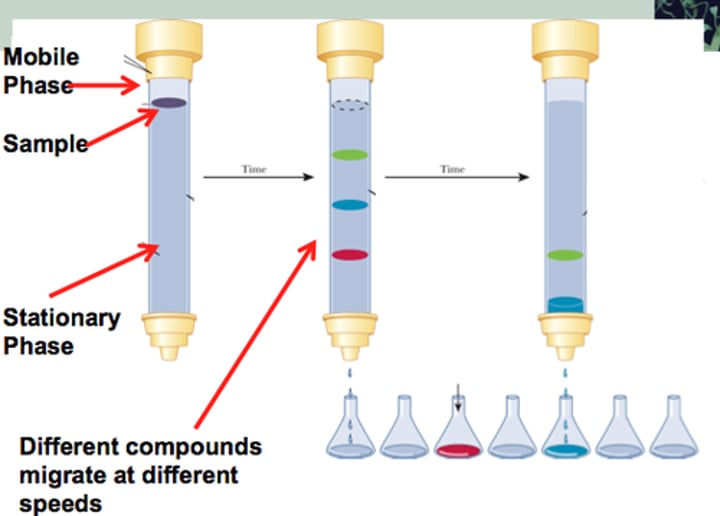
stationary and mobile phase of column chromatography:
- stationary phase: filled w/ silica gel (similar to TLC) (polar compounds elute LAST)
- mobile phase: with the solvent (nonpolar compounds elute FIRST)
which compounds elute first/last in column chromatography?
- nonpolar compounds elute FIRST b/c they easily travel past polar stationary phase
- polar compounds elute LAST b/c they retain in column d/t interactions w/ silica gel
high performance liquid chromatography (HPLC):
used to separate LARGE amounts of high molecular weight compounds via POLARITY (collection method - similar to column chromatography)
normal phase HPLC:
- stationary phase: polar (silica gel) - polar compounds elute LAST
- mobile phase: nonpolar (solvent) - nonpolar compounds elute FIRST
reverse phase HPLC:
the OPPOSITE of normal HPLC (b/c stationary = nonpolar)
- stationary phase: nonpolar (hydrocarbon chains fused on silica gel bands) - nonpolar compounds elute LAST
- mobile phase: polar (solvent) - polar compounds elute FIRST
Ion-exchange chromatography:
separate via charge states (+, -, or neutral) of mixtures of AAs, prots, or nucleotides
stationary and mobile phase of ion-exchange chromatography:
resin with anionic/cationic groups with counterions
counterions:
ions that are getting exchanged with chromatography
cationic exchange chromatography:
- have ANIONIC groups as resin (anions will elute FIRST in solution)
- cations elute last d/t interaction w/ anion resin
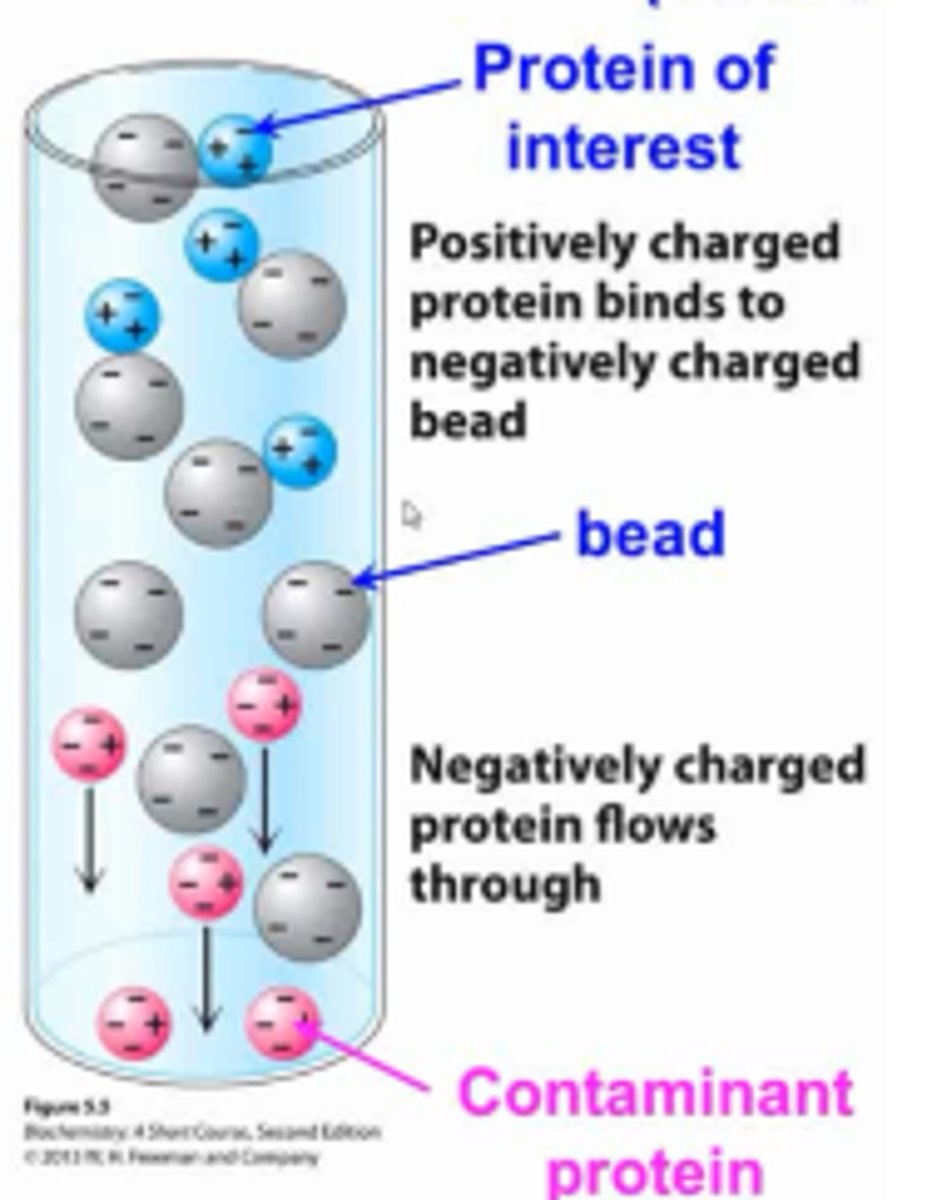
anionic exchange chromatography:
- have CATIONIC groups as resin (cations will elute FIRST in solution
- anions will elute last d/t interaction w/ cation resin
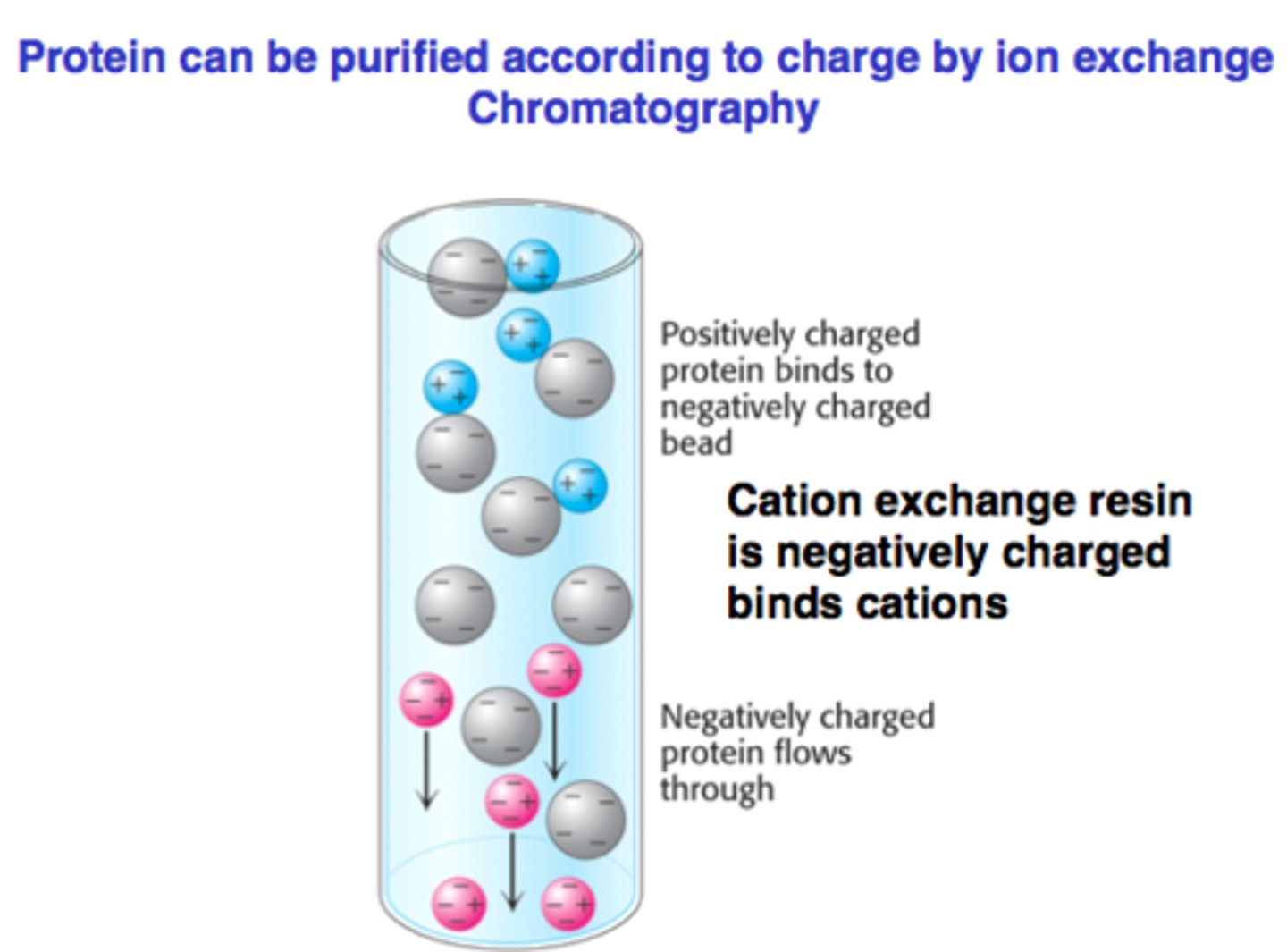
Affinity chromatography:
separation via lock-and-key interactions (ex: antigens and their antibodies) and is used to separate prots
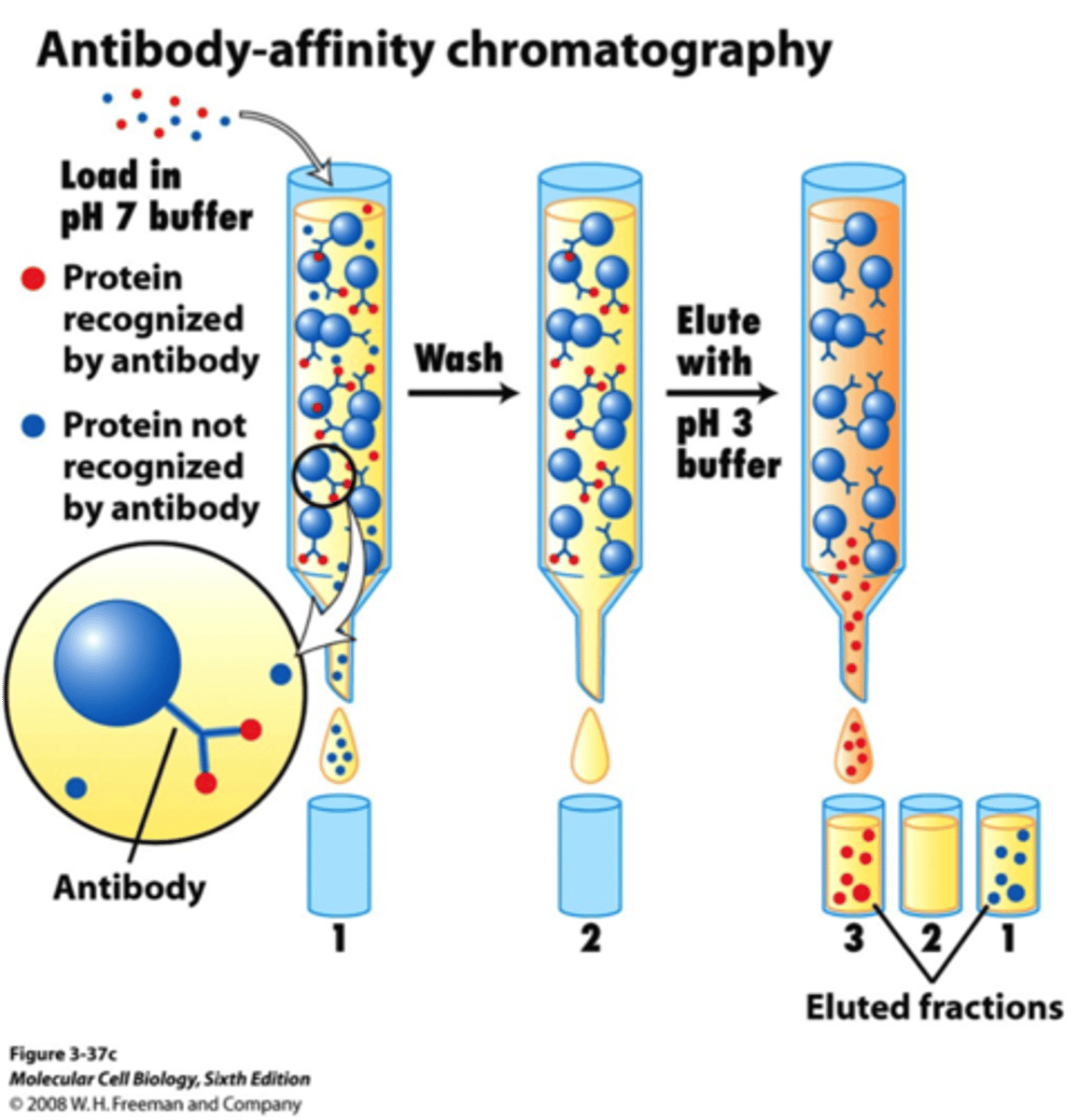
stationary phase of affinity chromatography:
small particles of resin linked to an antibody-binding protein (so that POI will bind and elute last)
gas chromatography (GC):
(analysis) used to separate SMALL amounts of low BP compounds based on volatility and BP
between water and CH2(CH3)2OH (isopropyl alc), what compound will elute first in GC?
isopropyl alcohol will FIRST b/c it has FEWER H-BONDS so therefore higher volatility
what is the relationship b/w volatility and BP?
high volatility = low BP
low volatility = high BP
elution order of GC:
lowest BP compounds (highest volatility) elute FIRST
highest BP compounds (lowest volatility) elute LAST
Distillation:
separation method based on volatility/BP of LARGE amounts of low BP (high volatility) compounds (collection method based on volatility/BP)
what intermolecular forces do BP measure?
H-bonds
dipole-dipole forces
london dispersion forces
factors that affect BP:
molecular weight and branching
how does molecular weight affect BP?
Heavier molecules have HIGHER BP b/c they're bigger with more surface area therefore more ability to bind to another molecule
how does branching affect BP?
more branches = lower BP d/t LESS surface area
simple distillation:
separate similar compounds based on their substances mixed in it and is used to remove impurities from a relatively pure liquid (based on VOLATILITY)
fractional distillation:
separating compounds with similar BPs using a column w/ packing material to increase surface area (only applicable when component BP diff is <30C)
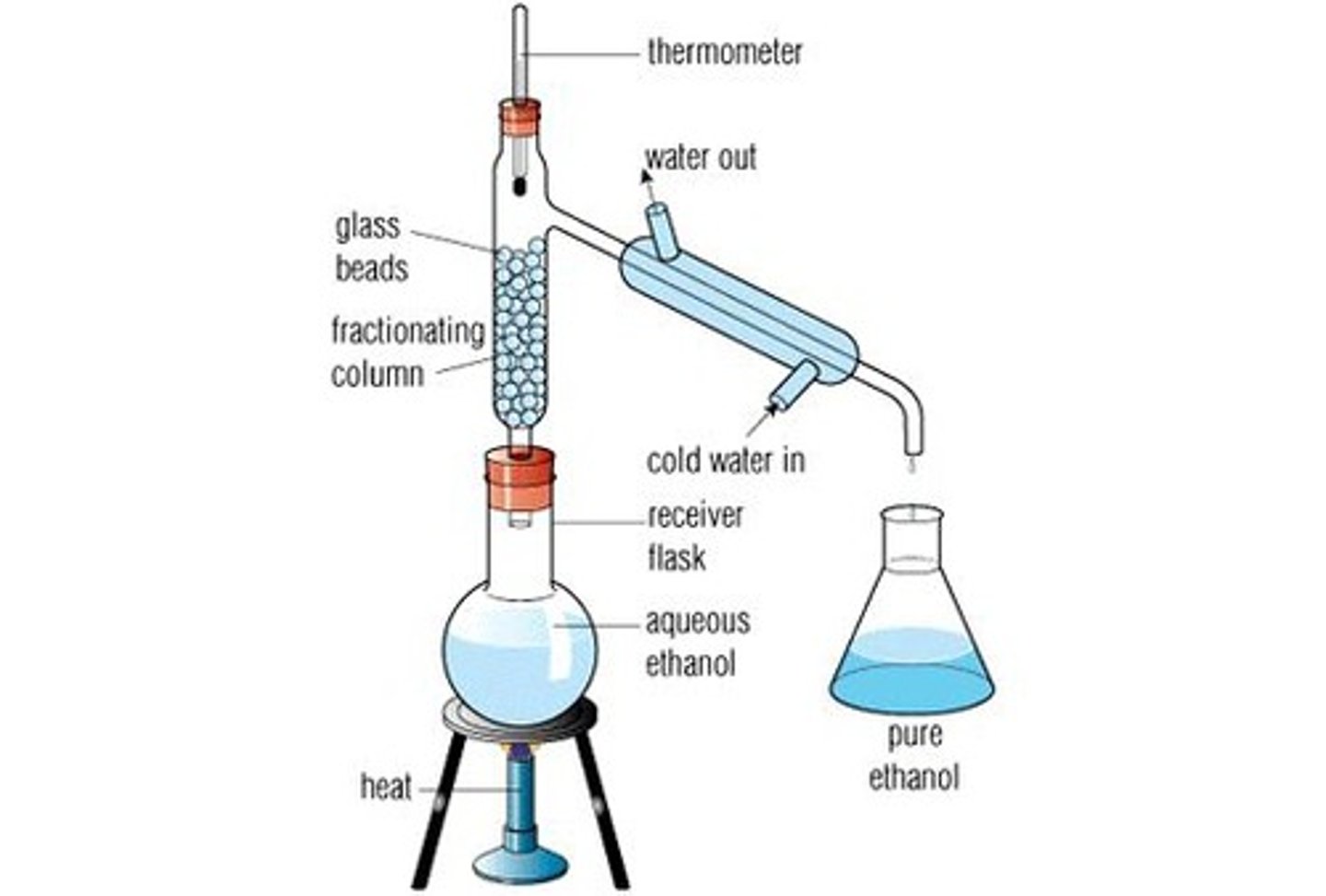
what is fractional distillation useful for separating?
diastereomers b/c they usually have similar polarity, size, etc. BUT BP IS A LITTLEE DIFFERENT
solvent extraction:
separates based on SOLUBILITY of compounds in polar/nonpolar solvents
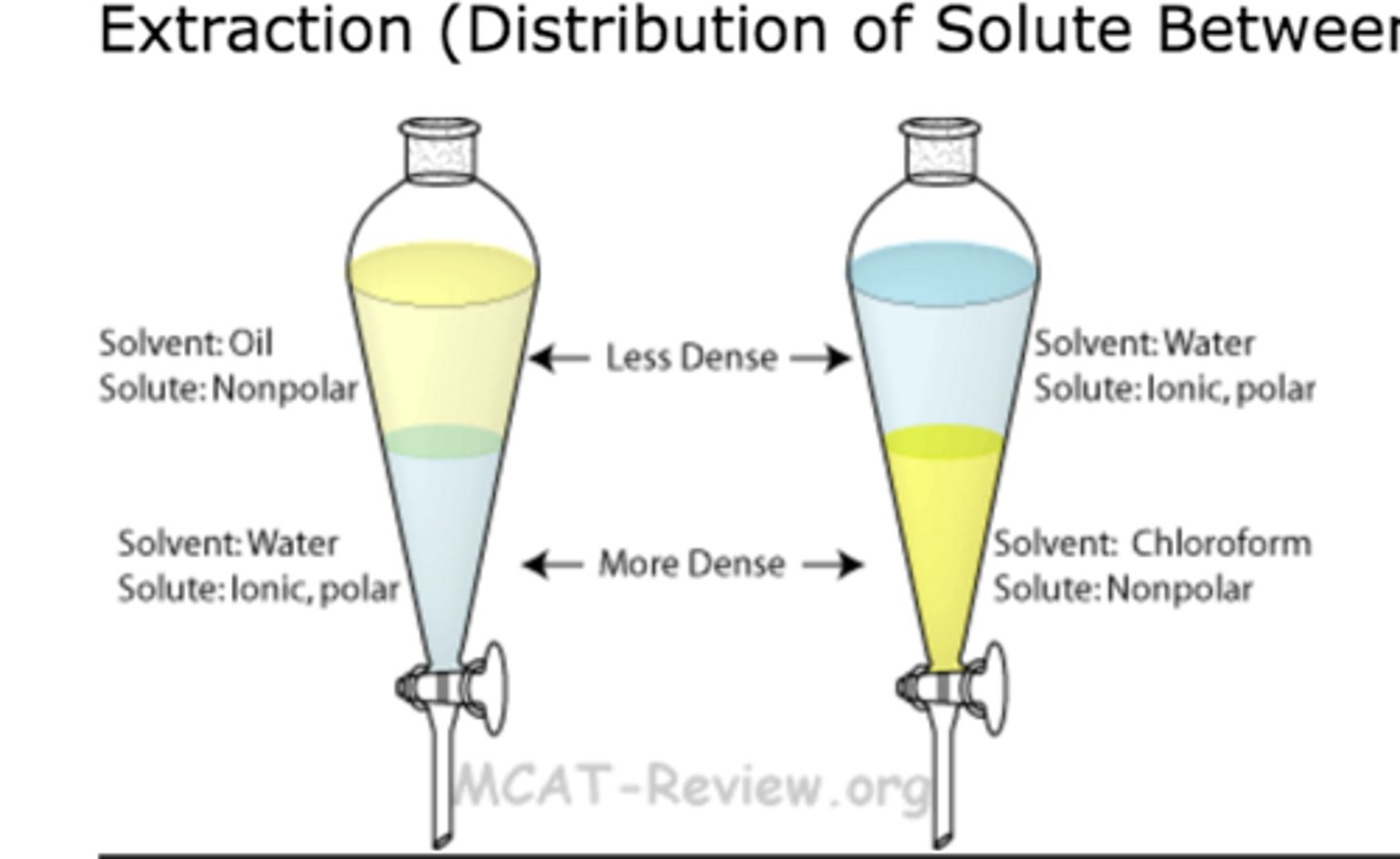
rules of solubility:
- like dissolves like
- compounds with less than/equal to 5C atoms w/ a polar group ARE water soluble
- compounds w/ >5C atoms need at least 2 polar groups to be water soluble
- charged functional groups dissolve readily in a polar solvent
which compounds are good for extraction?
ONLY phenols, carboxylic acids, and amines (b/c they are acidic/basic)
Are carbonyls and carboxylic acids good for extraction? Why/why not?
They are not bc they're not acidic enough to be pulled from organic layer into the aqueous layer
When acetone is reacted with CH3MgBr/ether followed by an acidic workup, which of the following is true with regards to the IR spectra?
A. The appearance of a broad peak at 3500 cm-1 and a sharp peak at 1715 cm-1
B. The disappearance of a broad peak at 3500 cm-1 and a sharp peak at 1715 cm-1
C. The disappearance of a broad peak at 3500 cm-1 and the appearance of a sharp peak at 1715 cm-1
D. The appearance of a broad peak at 3500 cm-1 and the disappearance of a sharp peak at 1715 cm-1
D. alcohol (-OH) has a stretch of 3300-3400 cm-1. And since ketone was used to make this alcohol, it disappears. Ketone spectrum is 1710-1720cm-1
In which of the following molecules does NMR spin-spin coupling occur?
A. BrCH2CH2Br
B. CH3CHBr2
C. CH4
D. (CH3)3CBr
B.
spin-spin coupling is splitting in H-NMR. CH3CHBr2 is the only choice with more than one set of hydrogens that are three bonds apart.
Which of the following molecules will have an infrared stretch nearest to 2250 cm-1?
A. (CH3)2CO
B. CH3CN
C. H2C=CH2
D. CH3OH
B.
The region from 2100-2300 cm-1 of the infrared spectrum shows the characteristic absorptions of C≡C and C≡N. Of the molecules listed, only CH3CN contains a triple bond.
Which of the following molecules will have an infrared stretch nearest to 1700 cm-1?
A. CH3-Ph
B. CH3CH2OH
C. CH3CH2CH2OCH3
D. CH3CH2CHO
D.
The region from 1650-1750 cm-1 of the infrared spectrum shows the characteristic absorptions of C=O double bonds. Of the molecules listed, only CH3CH2CHO contains a carbonyl.
Which of the following molecules would be expected to be the most soluble in water?
A. CH3(CH2)5CO2H
B. CH3CH2CO2H
C. HCO2H
D. CH3(CH2)3CO2H
C.
All of these molecules contain the carboxylic acid functional group, which can hydrogen bond and also ionize to increase its solubility in water. The choices differ only in the length of the carbon chain attached to the acid. Since saturated carbon chains are hydrophobic, the shortest-chain carboxylic acid will be the most water soluble, which is formic acid, HCO2H.
An amino acid has an isoelectric point of 6.0. Which one of the following is the best buffer pH that would facilitate isolating the amino acid from a mixture of amino acids using anionic-exchange chromatography?
A. 7.4
B. 5.5
C. 6.0
D. 4.8
A. (since you know 6.0 is wrong, any pH lower than that can't be right so it has to be 7.4 babes)
In order to get the best binding, the amino acid needs to be in a solution where it will have the most negative charge. When the pH = pI, the amino acid will be in its zwitterionic form and have an overall neutral charge, so a pH of 6.0 is incorrect. However, in a more basic environment (when pH > pI) the amino acid will be deprotonated, giving it an overall negative charge and making a pH of 7.4 the best choice. In a more acidic environment (pH < pI) the reverse will be true, and the amino acid will have an overall positive charge, so a pH of 4.8 or 5.5 will be too low to be effective.
Which of the following statements regarding size-exclusion chromatography is false?
A. Polarity changes of the mobile phase have negligible effects on elution rates.
B. Variation of the pore size of the stationary phase can be used to enhance separation.
C. Surface interactions on the stationary phase decrease elution rates
D. The smallest molecules in a sample are the last to elute.
C.
One factor that does not influence elution rates in SEC is the interaction of the mobile and stationary phases. Thus, changing the polarity of the solvent will not affect elution rates.