IMED1004 - Cell Cycle Control and Apoptosis (L29, L30)
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
52 Terms
Balance between cell loss and cell proliferation
- Adult tissues composed mostly of differentiated cells
- Constant low-level turnover of these cell
- Cells die & are replaced
- Stem cells proliferate & differentiate to replace dead cells (asymmetric mitosis)
- Some cell loss is accidental, other cell loss is through programmed cell death (apoptosis).
- Cell is abnormal in some way e.g., dividing too rapidly, irreparable DNA damage, infected by virus.
- One of roles of apoptosis is to survey for cellular abnormalities & execute self-destruct mechanism when detected. Cell loss not a problem as long as cell population is replenished (homeostasis).
Cell Cycle Control
PRECISE CONTROL DURING DEVELOPMENT AND GROWTH:
- crucial - determines size and shape of organs/tissues
.
- cell division - controlled by complex network of signalling pathways - extracellular signals and intracellular cues
.
- for homeostasis, cells continually assess own internal status to determine whether conditions appropriate to initate apoptosis in cells that fail to successfully complete some phase of the cell cycle
Cell cycle regulatiory mechanisms
- discovered in yeast, worms, xenopus, clam and sea urchin - also operate in somatic cells of humans: well conserved (important)
- loss of control/disturbance in the cell cycle cancer (kills 1/6 people in the developed world
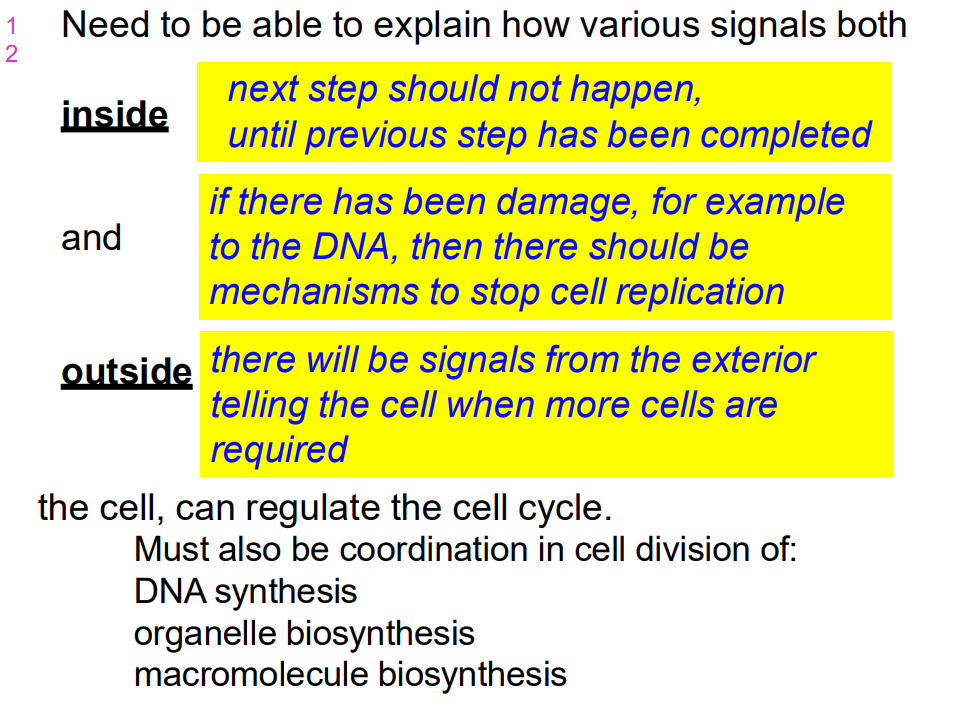
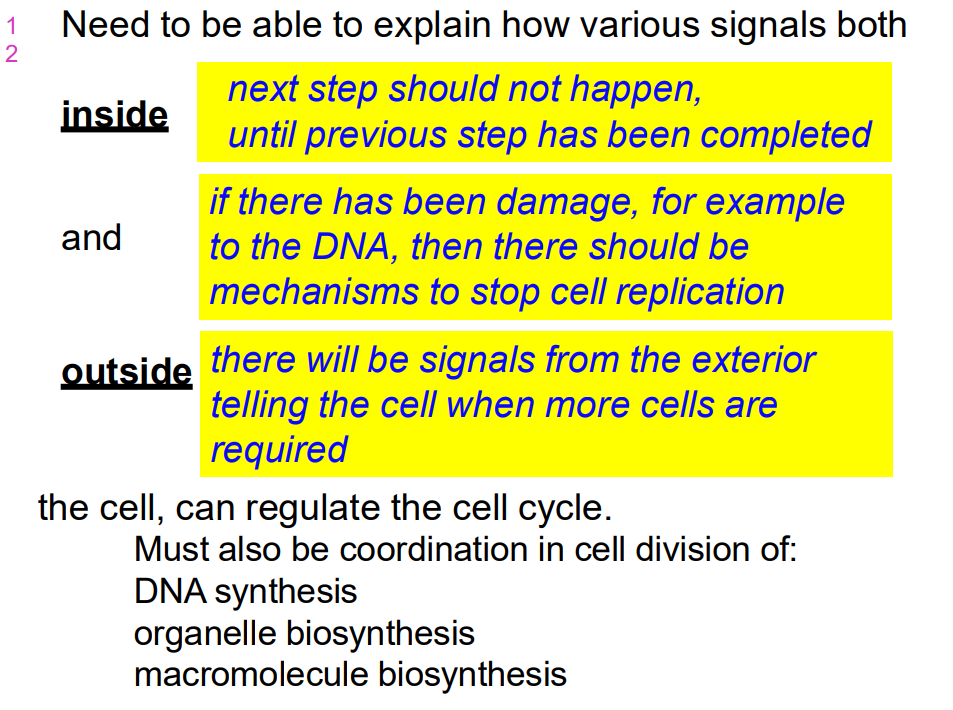
Need to be able to explain how various signals both inside and outside the cell can regulate the cell cycle
- must also be coordination in cell division of:
- DNA synthesis, organelle biosynthesis and macromolecule biosynthesis
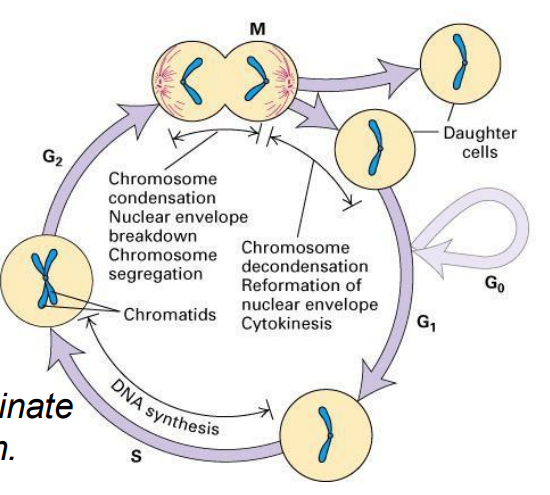
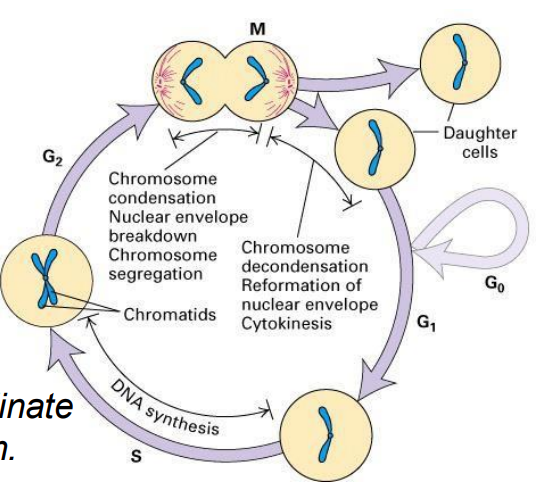
Cell Cycle (CC)
- a cyclic process of duplication of cell contents then division to produce 2 daughter cells, then duplication, then division
- the process is highly regulated
- want to emphasise that the core of this system is an ordered series of biochemical switches
- dividing cells must coordinate all aspects of their growth
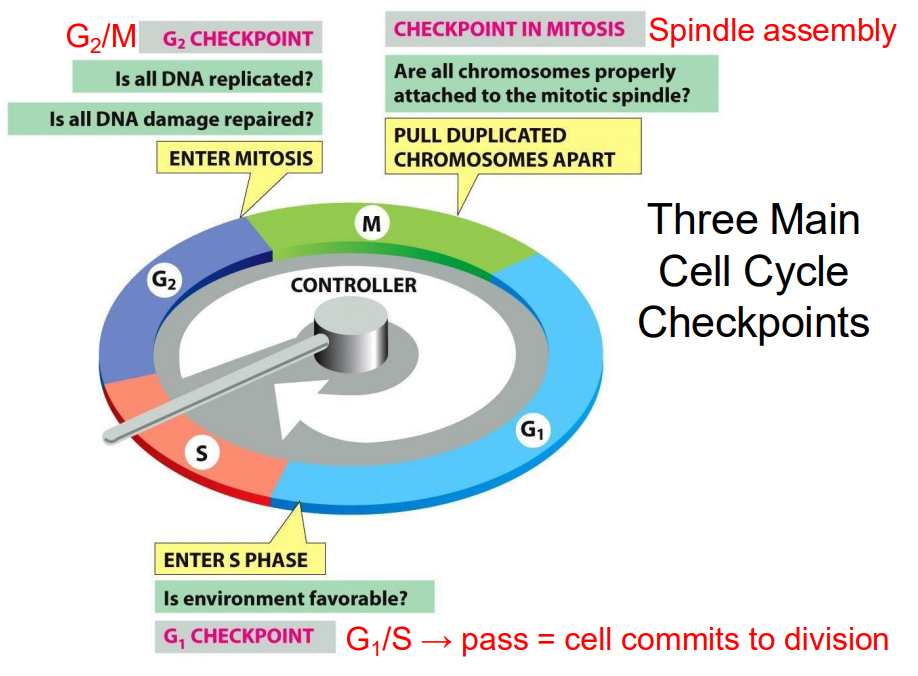
Three Main Cell Cycle Checkpoints
- these are particular points of control where a decision can be made
- G2/M checkpoint: Is all DNA replicated? Is all DNA damage repaired? Enter Mitosis
- Checkpoint in Mitosis: are all chromosomes properly attached to the mitotic spindle. Pull duplicated chromosomes apart
- G1 Checkpoint: Is environment favourable? Enter S phase
.
G1/S -> pass = cell commits to division
Checkpoints in the cell cycle
CHECKPOINT CONTROLS ENSURE THAT:
- chromosomes are present and that critical stages of cell cycle are completed before the next stage begins
- entry into S or M is prevented if DNA is damaged
- spindles are properly formed and chromosomes are properly attached
- cellular environment is favourable
.
- SO, what sets up these checkpoints
What controls passage through the cell cycle
- A small group of heterodimeric protein kinases regulate the cell cycle - thus, regulated phosphorylation is critical (regulated degredation also important)
- These are CDK-cyclin complexes
.
1ST SUBUNIT, REGULATORY SUBUNIT = CYCLIN
- Levels of cyclins increase and decrease during the cell cycle
2ND SUBUNIT, CATALYTIC SUBUNIT = CYCLIN-DEPENDENT PROTEIN KINASE (Cdk)
- Cdk have kinase activity, but only when bound by appropriate cyclin... cyclin NOT only activates protein kinaes activity, but also determines substrates (targtes) to be phosphorylated
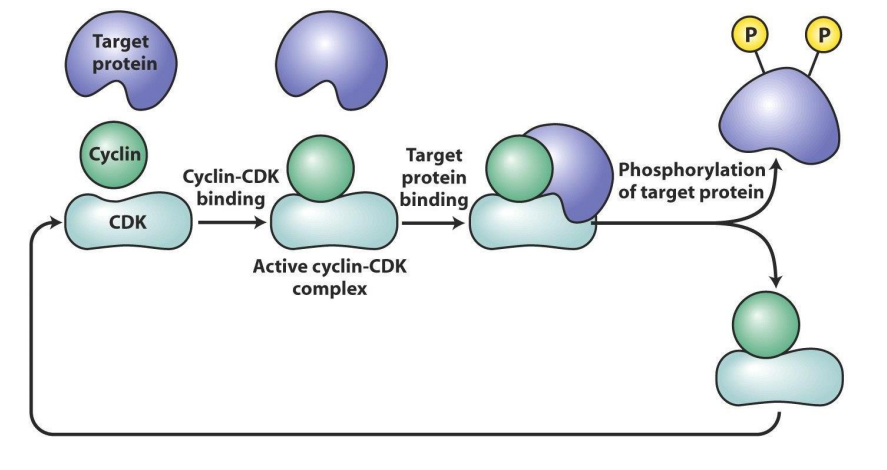
How Cdk works
- target protein (substrate) binds to cyclin part of active Cdk-cyclin complex, which places target phosphorylation site/s in active site of Cdk subunit
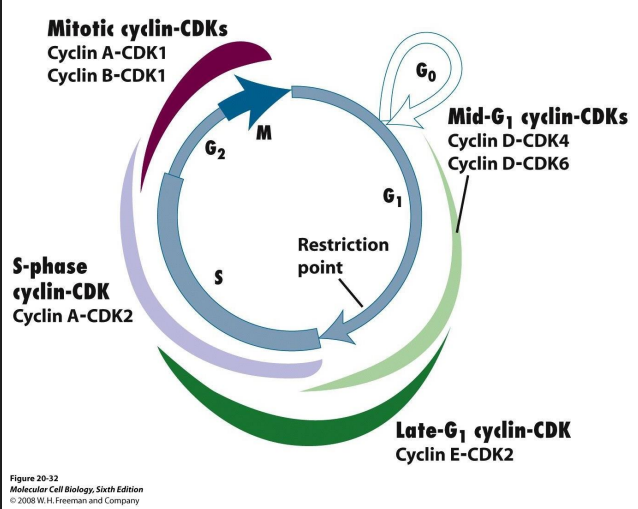
Cell cycle is complicated: multiple cyclins and Cdk various combinations in different phases of CC
NOTE:
- Cyclins are a family: A, B, D, E. Their levels change
throughout the cell cycle
.
Cdks are a family too: Cdk1, Cdk2, Cdk4, Cdk6
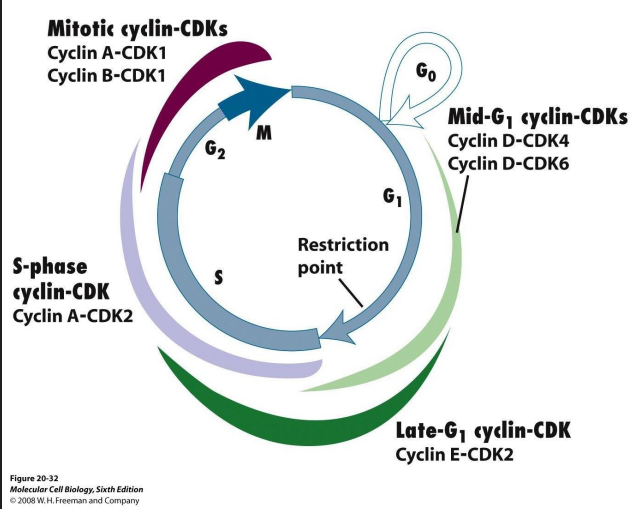
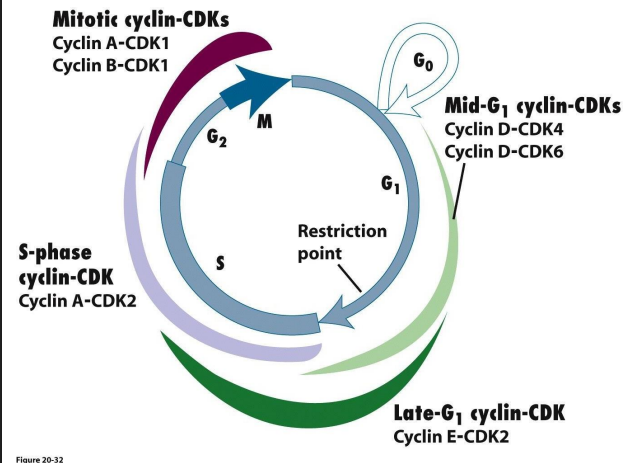
More detail on Cdk
- engines that drive cell cycle from one step to the nxet are these protein complexes of Cdk-cyclins
- going to the next step in cycle requires activation of genes whose protein products are necessary for next phase
- Activation occurs through turning on trasncription factors by Cdk-cyclin complexes
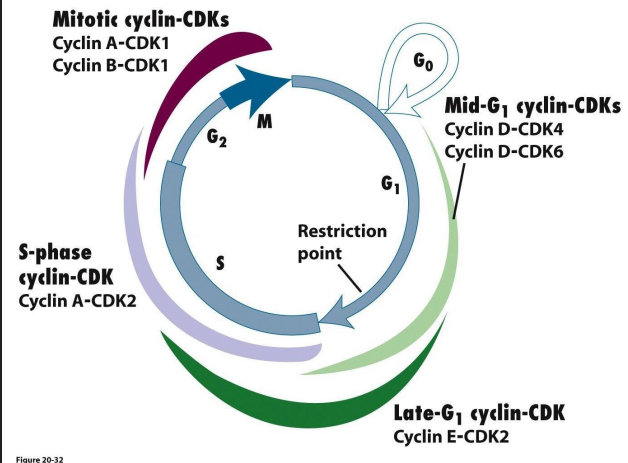
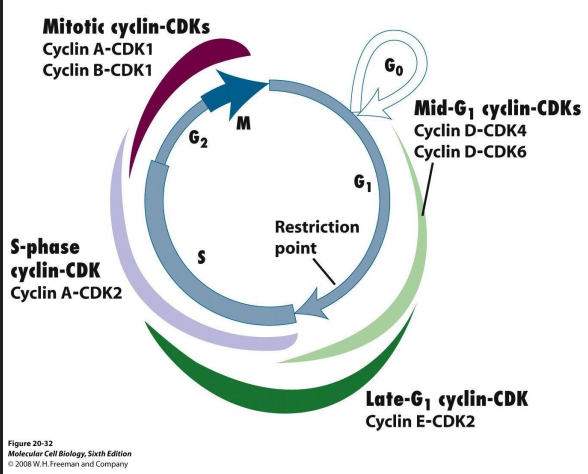
Cdk-cyclins during G1
- takes cells into S phase
- G1 Cdk-cyclin complex activates components
- TF turns on genes encoding DNA polymerase
- Genes for enzymes produce dNTPs
- proteins involved in dupliation of chr.
- genes for subunits of next Cdk-cyclin complex
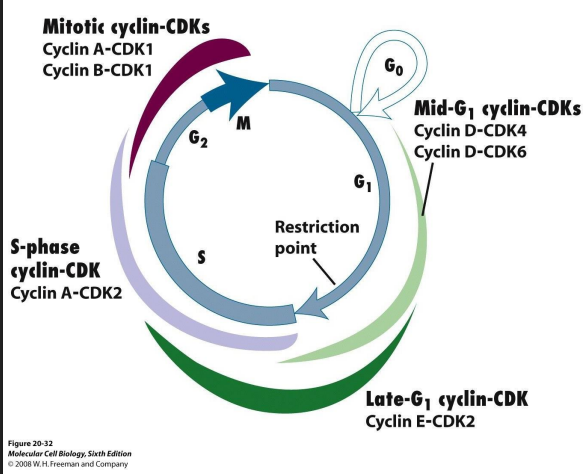
Cell Cycle Regulation Sequential activation
- sequential activation of different Cdk-cyclin complexes controls cell cycle progression
- if an active Cdk-cyclin present at wrong time, it will cause inappropriate genes to be transcribed (or switched off)
- Cdk present throughout cell cycle, so which complex is active is a function of which cyclin is present
- as different cyclins present at different stages of cell cycle, each phase characterised by phosphorylation of different target proteins
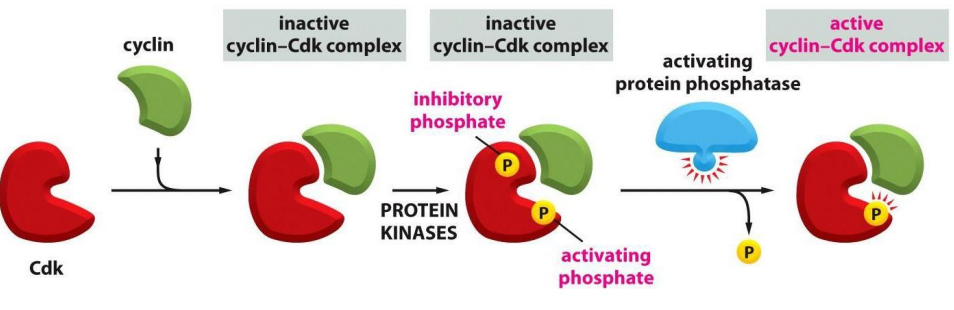
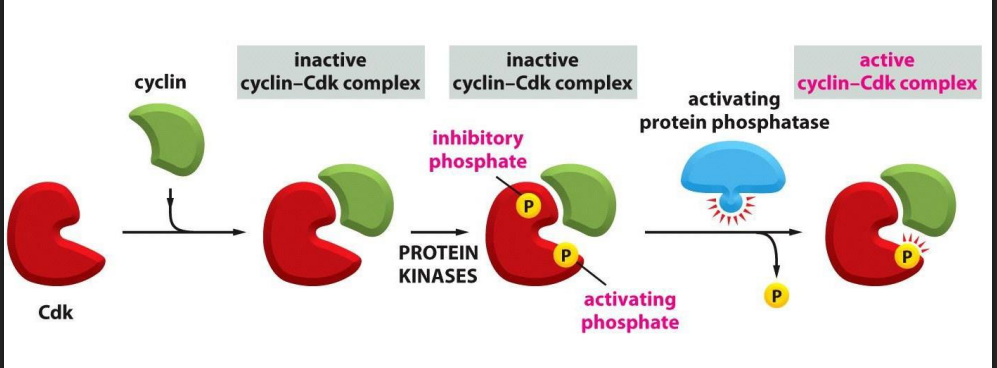
For a Cdk to be active, it must be
- bound by a cyclin AND
- phosphorylated at one site AND
- dephosphorylated at another site
.
- protein kinases phosphorylate the inactive Cyclin-Cdk complex
- removal of the inhibitory phosphate allows it to get activated
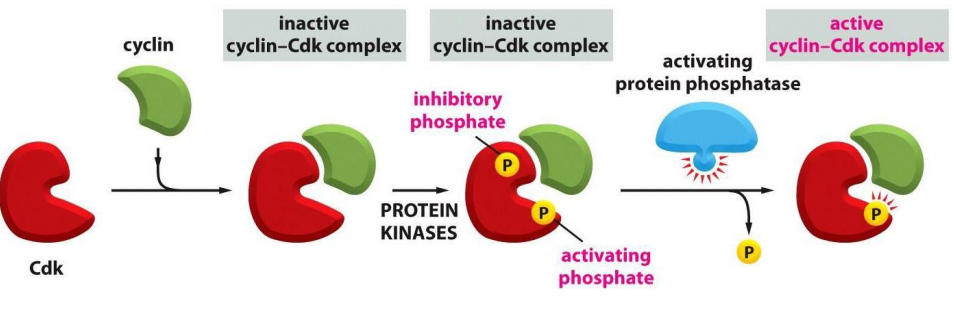
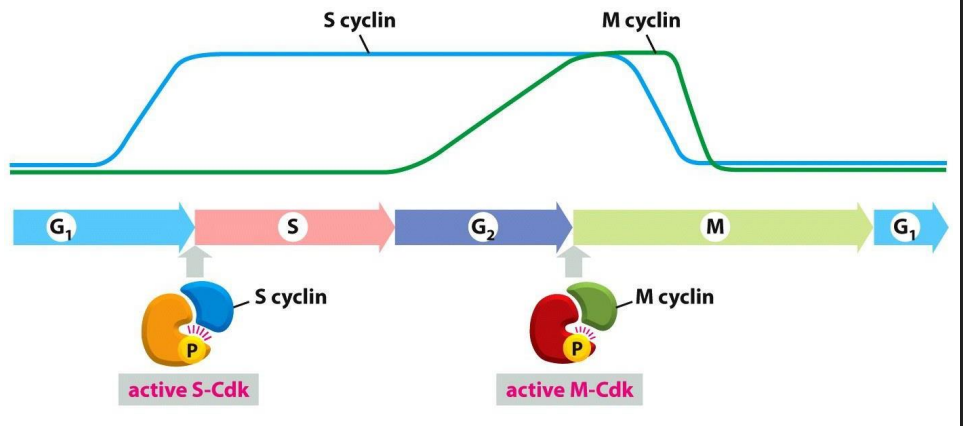
Distinct Cdks associated with different cyclins to trigger different events in cell cycle
ONLY TWO ARE SHOWN: ONE THAT TRIGGERS S PHASE, ONE THAT TRIGGERS M PHASE. Note also the activating P is present
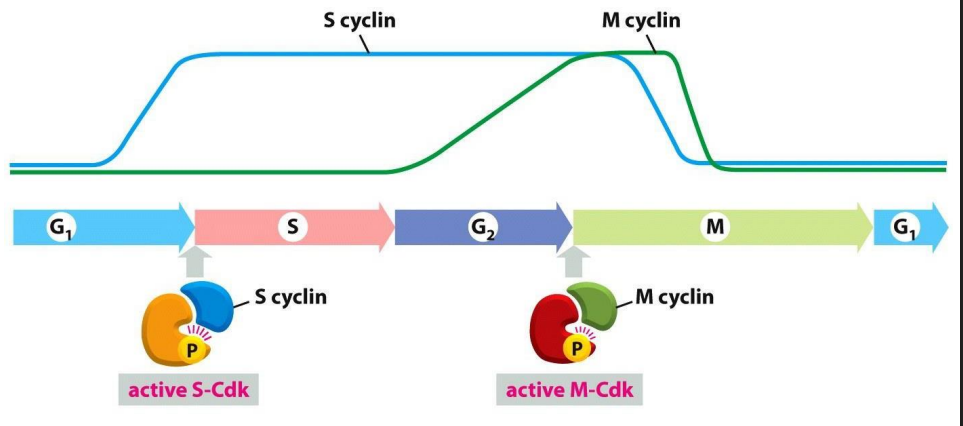
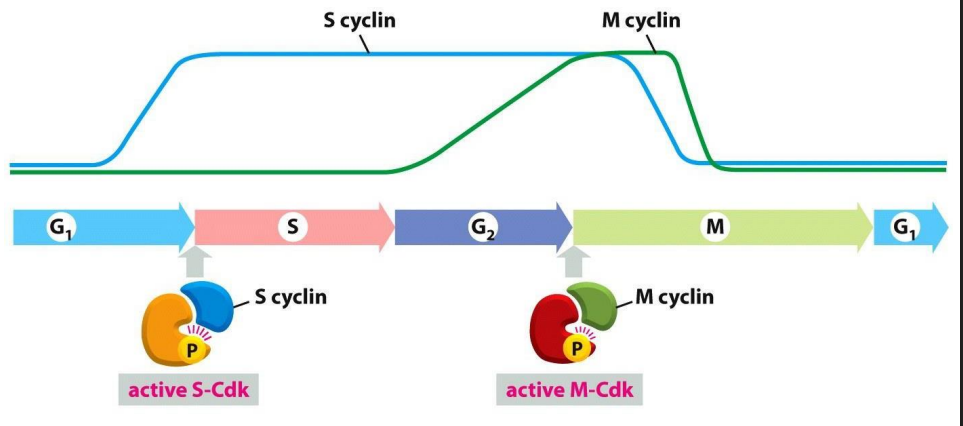
Progression through the CC can be controlled by turning off Cdk-cyclin activity: Cdk inhibition
- Molecular breaks provide "checkpoints" in the CC where progression is halted
- basically these complexes are like the checkpoints
Mammalian Cell Studies
- Cells in culture (in vitro), can be more readily studied for changes over time. Can look at changes in shape (morphology) & organelle changes (e.g. mitosis).
- If starved, or growth factors are absent, then cells remain in G1 ; if extended time in G1 , then cells move into G0
- If cells are stimulated by growth factors (usually present in foetal bovine serum used in vitro), then cells move into S phase.
- Once this happens, cells will complete a cycle in about 24 hours (depending on cell type).
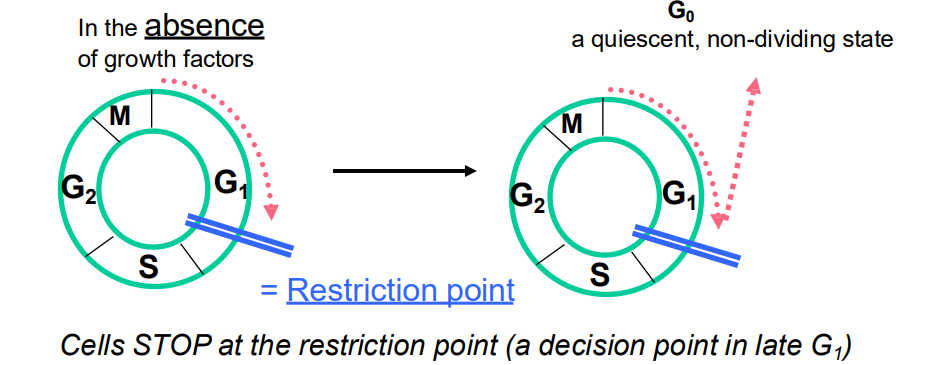
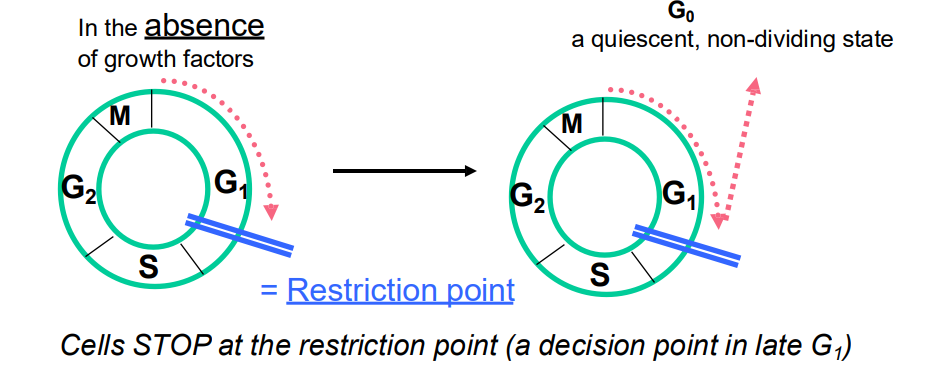
Regulation of Cell Cycle by Extracellular Signals
- Cells stop at the restriction point (a decision point in late G1)
- But ADD GROWTH FACTOR, cells will pass through the restriction point and are committed to entering CC and to completing cell cycle (unless stopped by later checkpoints)
- so even if extracellular signals are removed, the cell cycle continues if the restriction point has been passed
.
- quiescent: not undergoing cell cycle
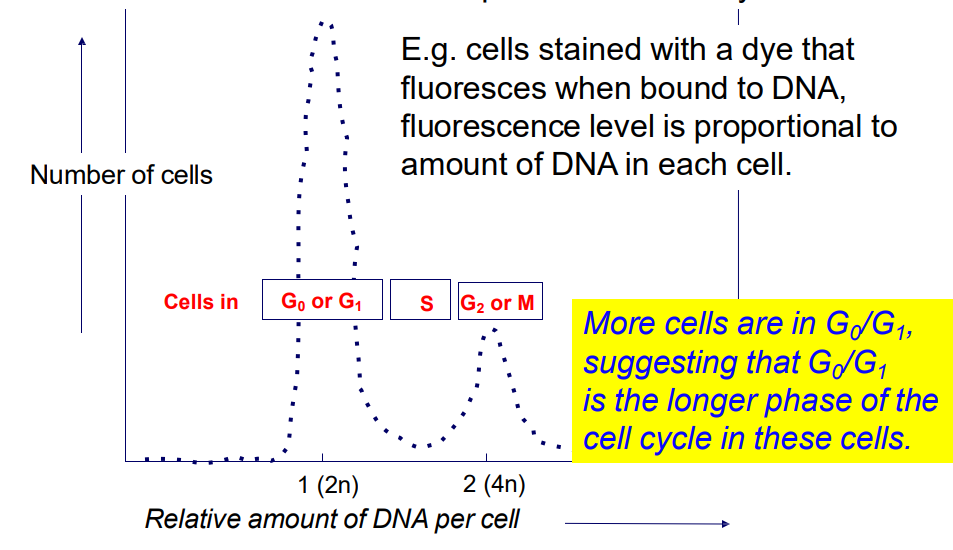
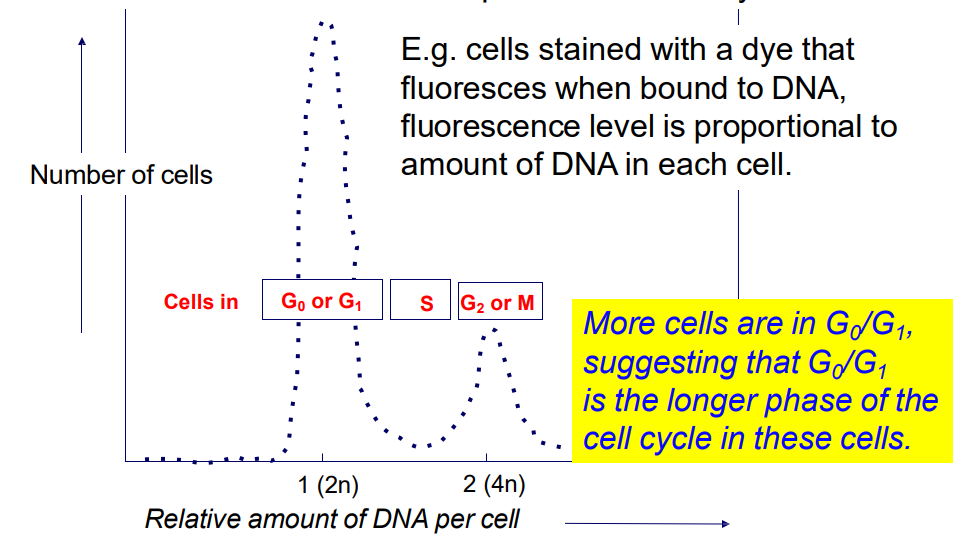
We can study cell progression through CC by looking at change in DNA content in the cell
2n -> 4n -> 2n (n = haploid)
- Analysis of DNA content by flow cytometry allows a "snapshot" of the number of cells in each phase of the
cell cycle
.
e.g cells stained with a dye, fluorescence level is proportional to amount of DNA in each cell
- more cells are in Go/G1, suggesting that G0/G1 is the longer phase of the cell cycle in these cells
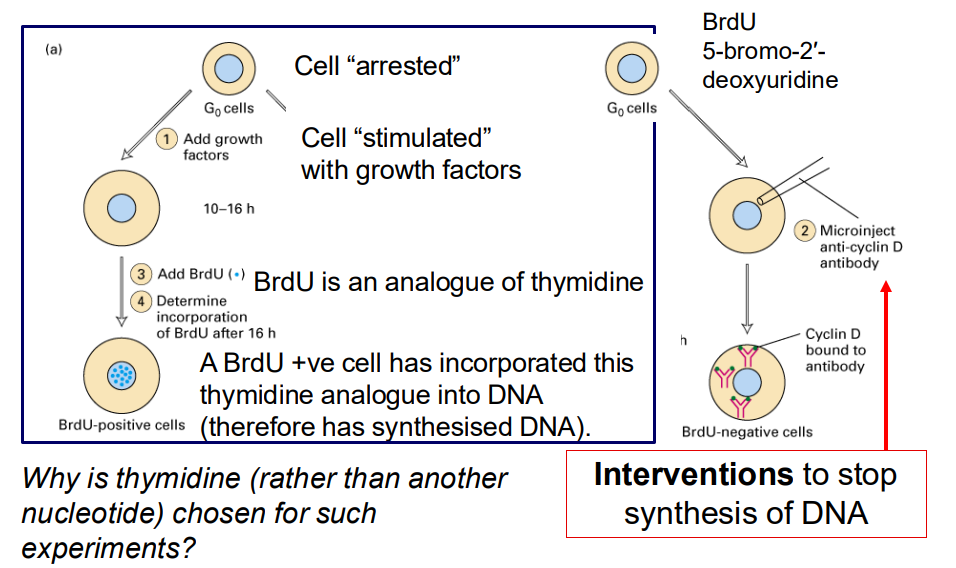
In addition to morphological or DNA content studies, can perform interventional studies to evaluate cell proliferation
- why is thymidine (rather than another nucleotide) chosen for such experiments
- interventions to stop synthesis of DNA
.
- cell "arrested"
- Cell "stimulated with growth factors"
- BrdU is an analogue of thymidine
- A BrdU +ve cell has incorporated this thymidine analogue into DNA (therefore has synthesised DNA)
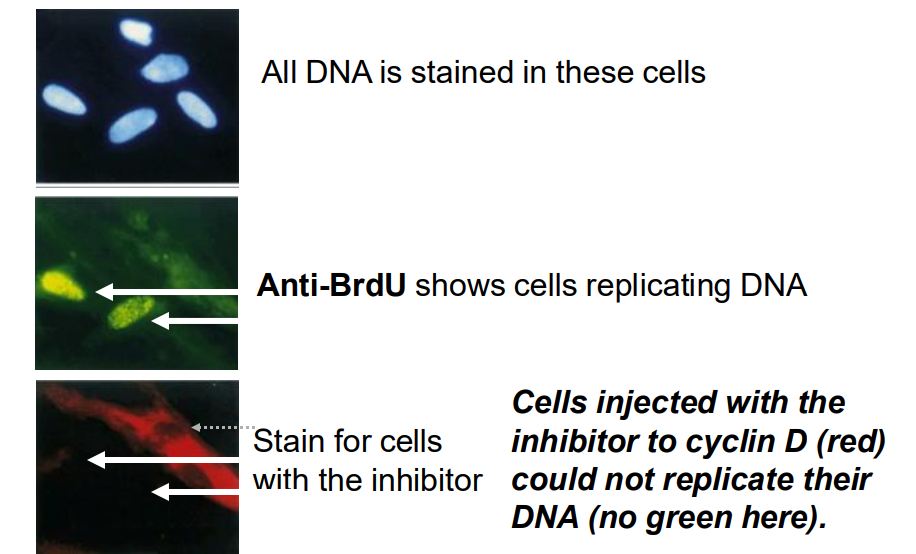
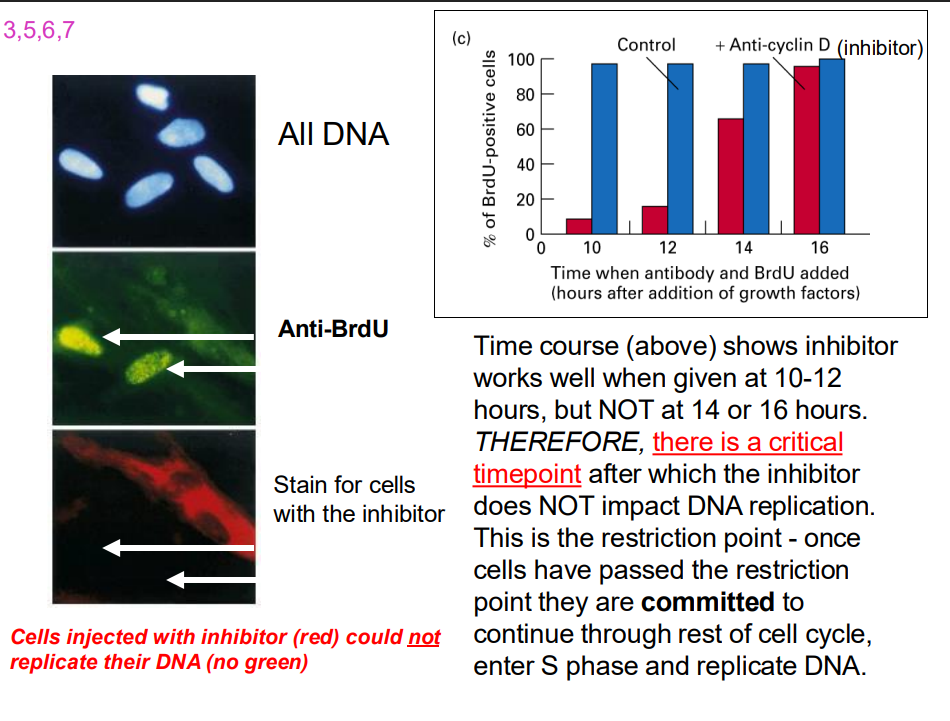
Results from such an interventional experiments
- All DNA is stained in these cells
- Anti-BrDU shows cells replicating DNA
- Stain for cells with the inhibitor (cells injected with the inhibitor to cyclin D (red) could not replicate their DNA (no green)
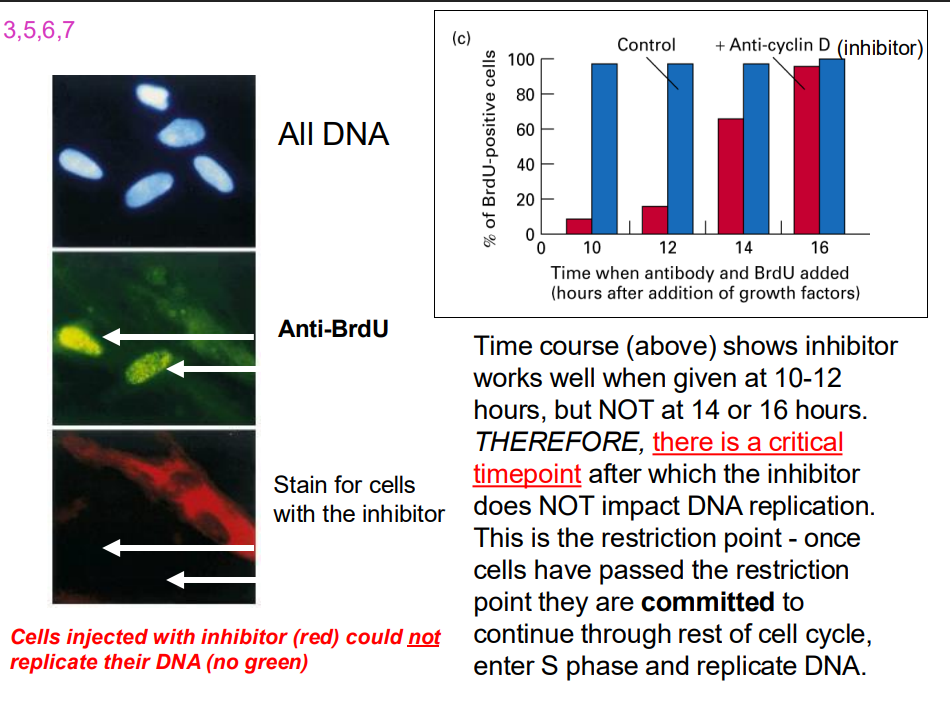
Time course experiment
- time course shows inhibtor works well when given 10-12 hours...
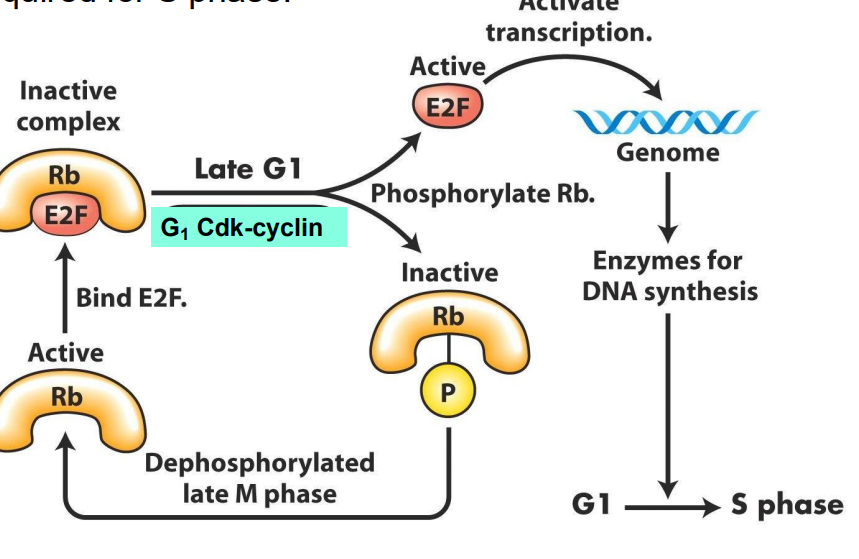
How do the growth factors act
Transcription occurs within a few hours, then translated into proteins: many of these proteins are also TFs (e.g E2Fs), some are cyclins
- These proteins and their mRNAs are unstable, so if withdraw growth factor, proteins are rapdily broken down and cells cannot pass the restriction point. Thus cannot enter S
- What are the biochemical actions of the newly synthesised G1 Cyclins and Cdk?
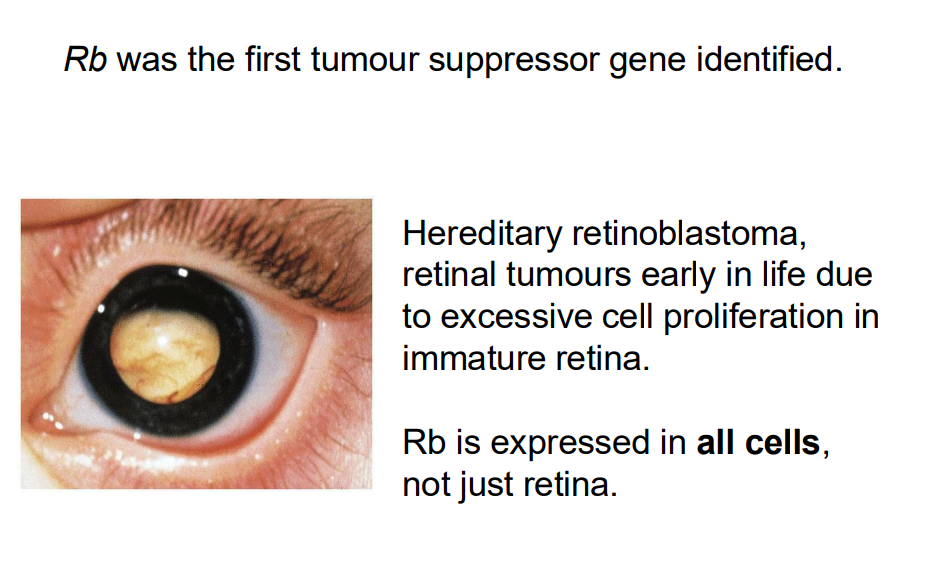
- these act to regulate phosphorylation of a protein = retinoblastoma protein (Rb)
In Non-proliferating cells, Rb is UNPHOSPHORYLATED
- in this state, it binds to the transcription factor, E2F, trapping E2F in the cytosol
- thus, E2F cannot bind DNA, and enhance transcription
- in proliferating cells, from late M phase through middle of G1, Rb sequesters (holds) E2F in protein complex in cytosol, so E2F does not promote transcription.
- G1 to S transition: late G1, active Cdk-cyclin complexes produced which phosphorylate Rb
- Phosphorylation of Rb protein inhibits its binding (and thus its repression) of E2F, which is released from complex, moves into nucleus - transcriptional activity
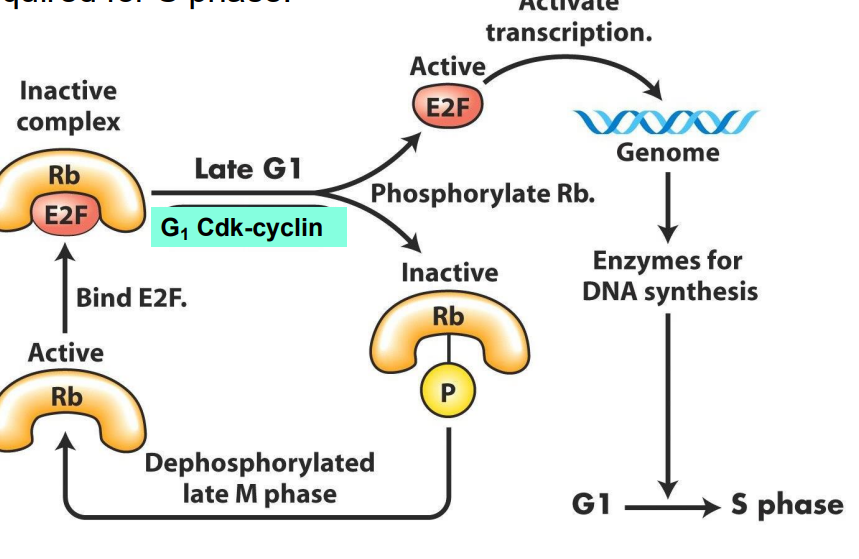
E2F free to promote transcription of genes vital for DNA synthesis and chromosome replication, AND of cyclins required for S phase
DIAGRAM ON SLIDE 29
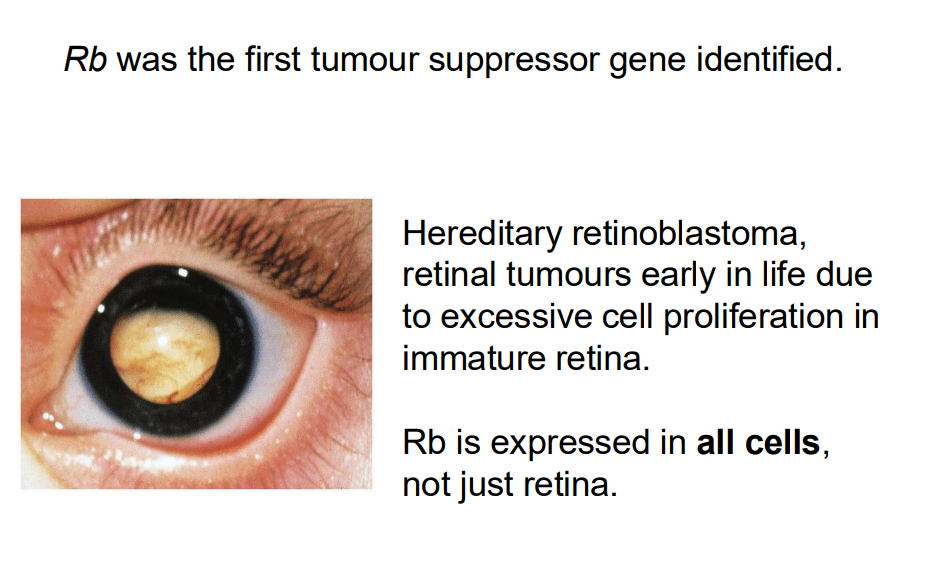
Rb was the furst tumour suppressor gene identified (NO LEARNING OUTCOEM)
DIAGRAM ON SLIDE 30 (whole slide)
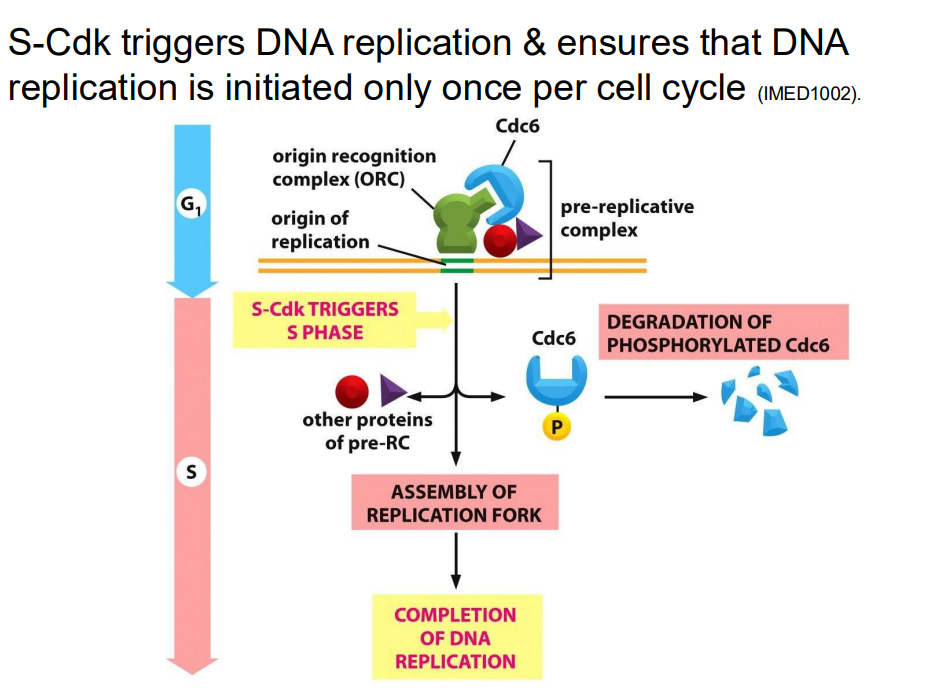
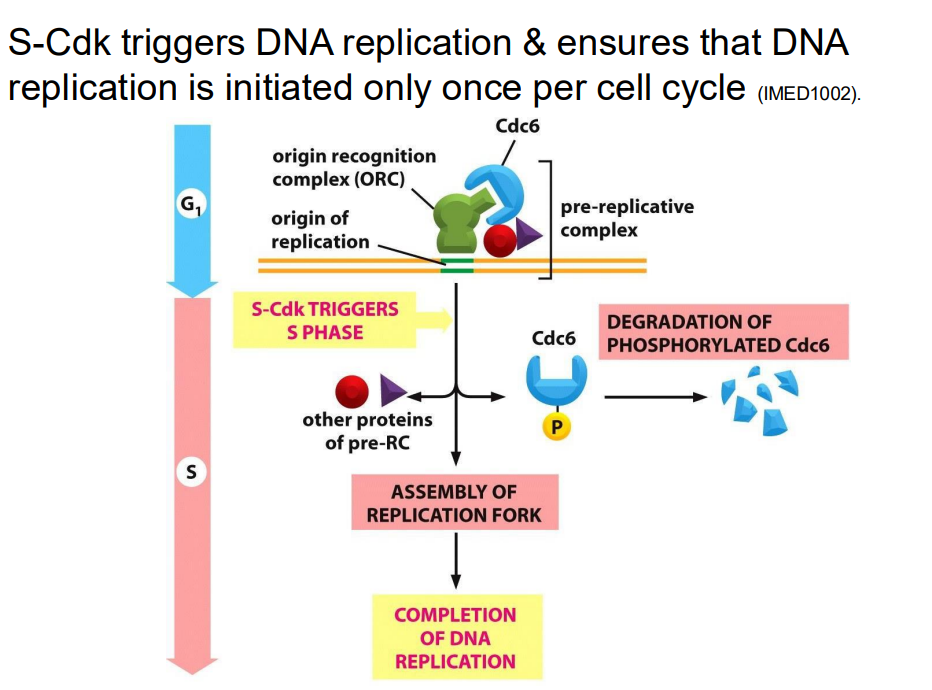
S-Cdk triggers DNA replication and ensures that DNA replication is initiated only once per cell cycle (NO LEARNINGOUTCOME)
DIAGRAM ON SLIDE 31
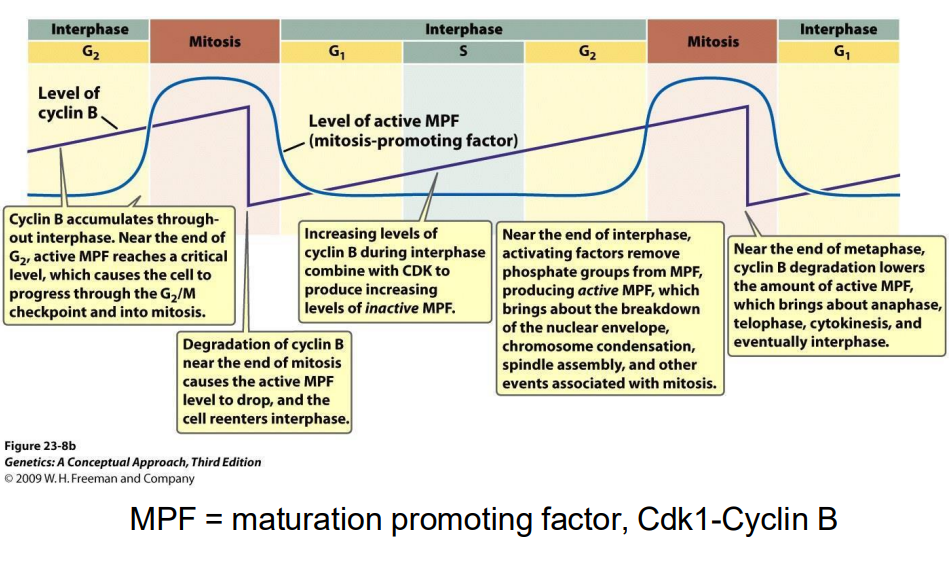
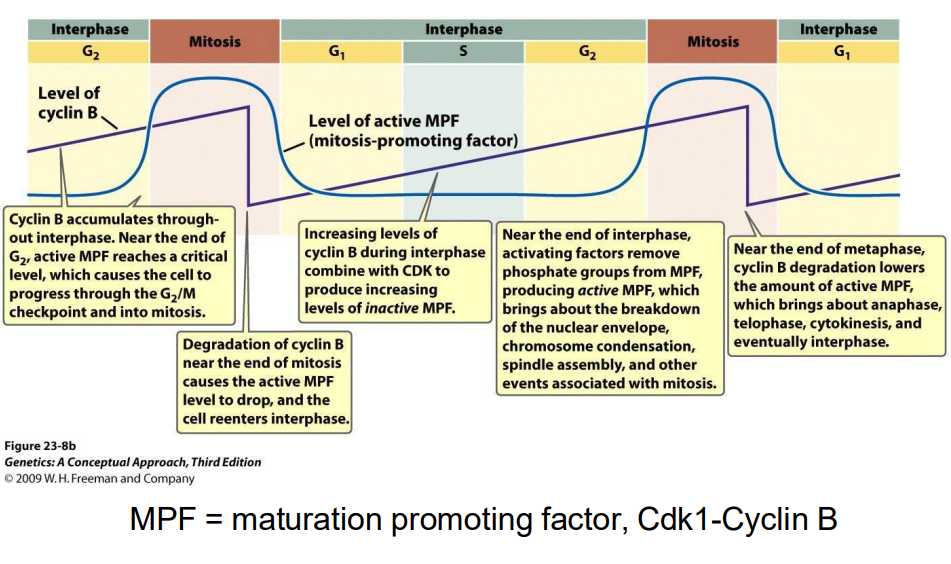
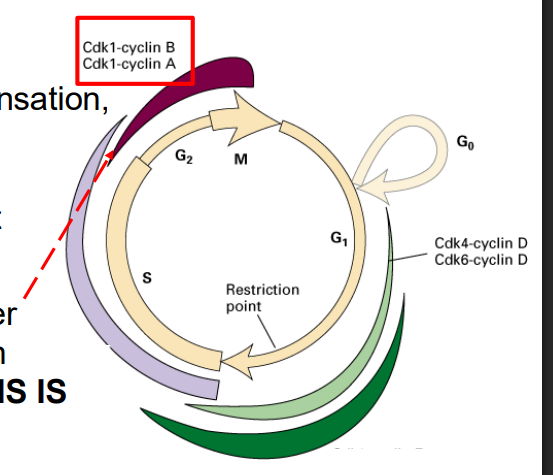
G2-M transition regulated by Cyclin B
- MPF = maturation promoting factor, Cdk1-Cyclin B (in the pruple int he digram)
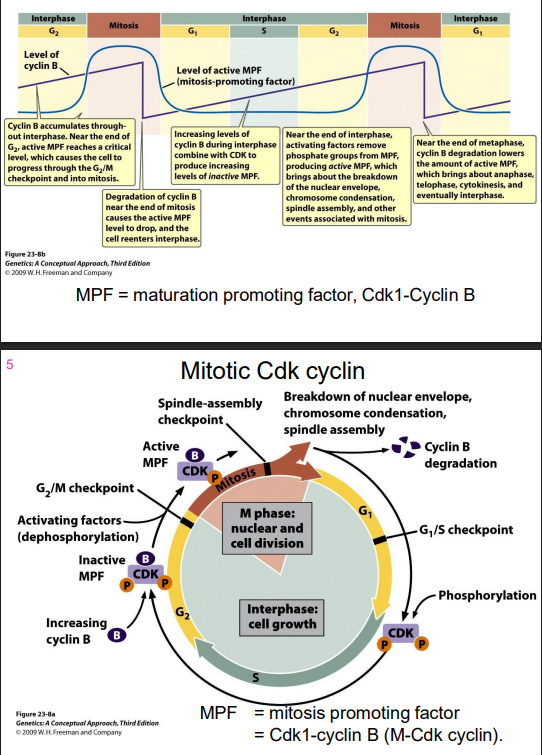
Mitotic Cdk cyclin
- MPF = mitosis promoting factor = Cdk1-cyclin B (M-Cdk-cylcin)
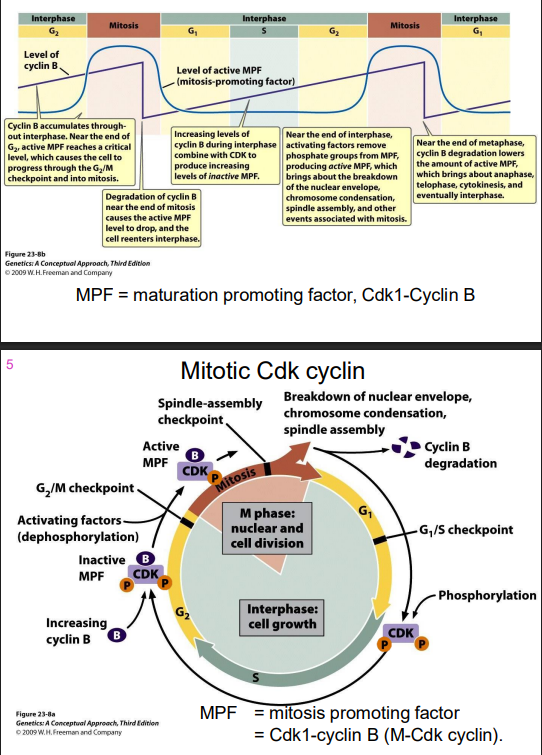
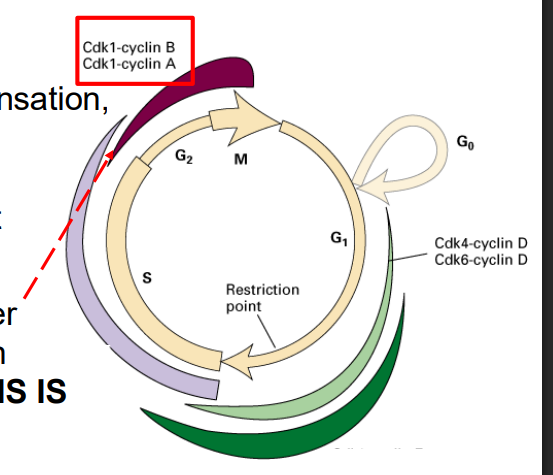
Cdk1 is required for entry into mitosis
Mitotic Cdk cyclin:
- activates chromosome condensation, nuclear envelope breakdown, spindle assembly, alignment of chromosomes at metaphsae plate
NOTE: this is synthesised earlier (S/G2), but its activity is held in check UNTIL DNA SYNTHESIS IS COMPLETE
.
there are also specific Cdk INHIBITORS in the cell.
- CKIs (Cyclin-Kinase Inhibitors): 2 families (Cdk inhibitory proteins (CIP) and Ink4 family (Inhibitors of Kinase 4)
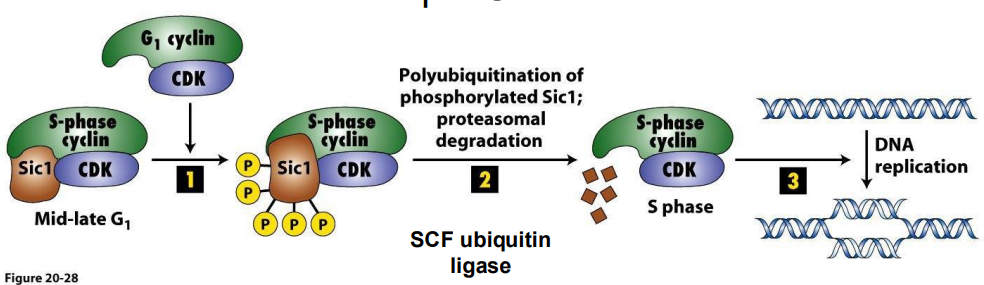
Example CIP = Sic1
- S Cdk-cyclins accumulate in G1, but are inhibited by Sic1, so held in check to prevent DNA replication until cell prepared
- Late G1 cyclin-Cdk phosphorylate Sic1, marking it for **polyubiquitination and degredation. Once CIP is degraded, remove CC inhibition, have active S-Cdk cyclins
.
**Ubiquitin = small regulatory protein with many functions, in this case, binding of ubiquitin causes protein degredation
.
- ubiquitination marks a protein for degredation
- basically upon depletion of Sic1 via ubiquitination, it then releases this complex to then performance functrion
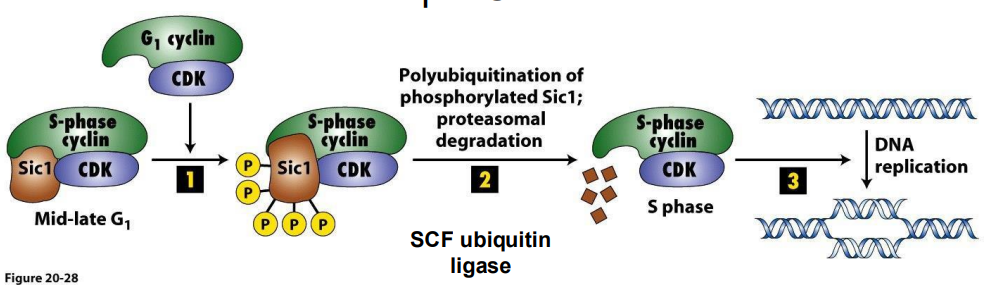
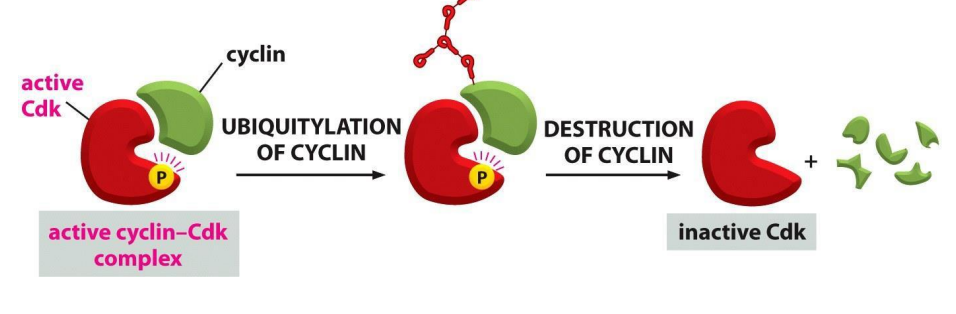
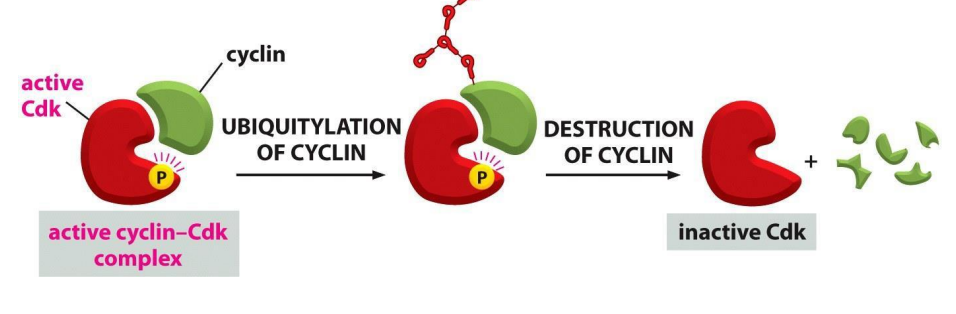
So, proteins that inhibit Cdk can arrest cell cycle at CC checkpoints
- the activity of Cdk is also regulated by cyclin degredation, for example by ubiquitination
.D
- basically ubiquitylation marks the complex for degredation
- ubiquitylation is stimulated by ubiquitin-protein ligases
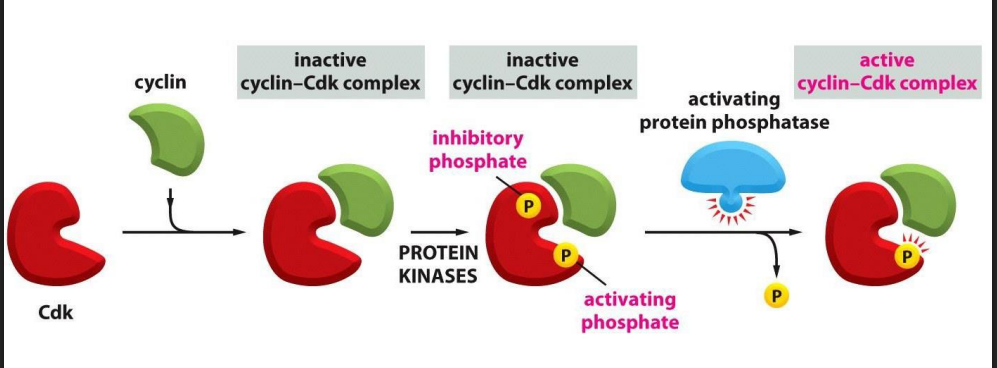
Recall: the activity of Cdk is also regulated by phosphorylation state
- multiple levels of control to ensure proper timing of activation, if this goes awry
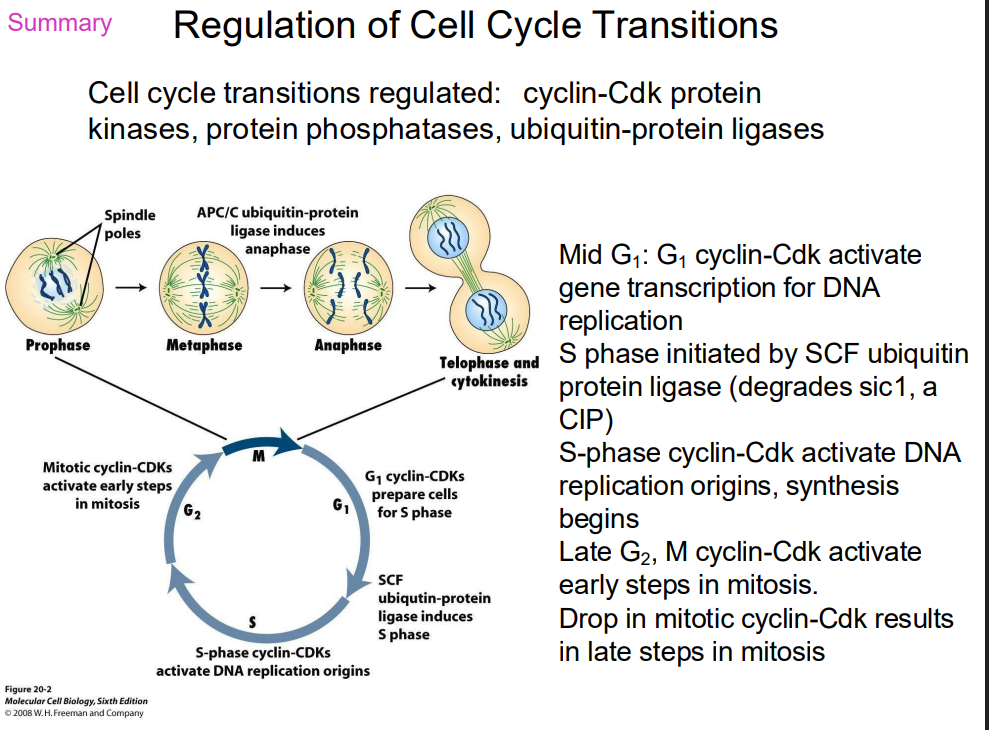
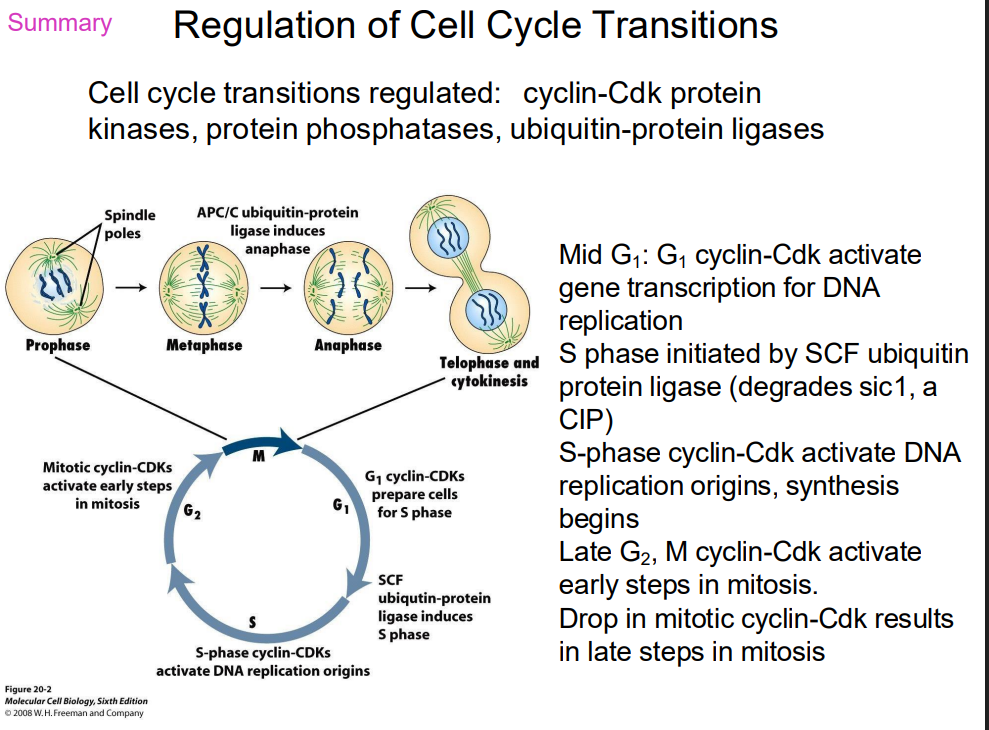
SUMMARY OF REGULATION OF CELL CYCLE TRANSITIONS
DIAGRAM ON SLIDE 38 (WHOLE SLIDE)
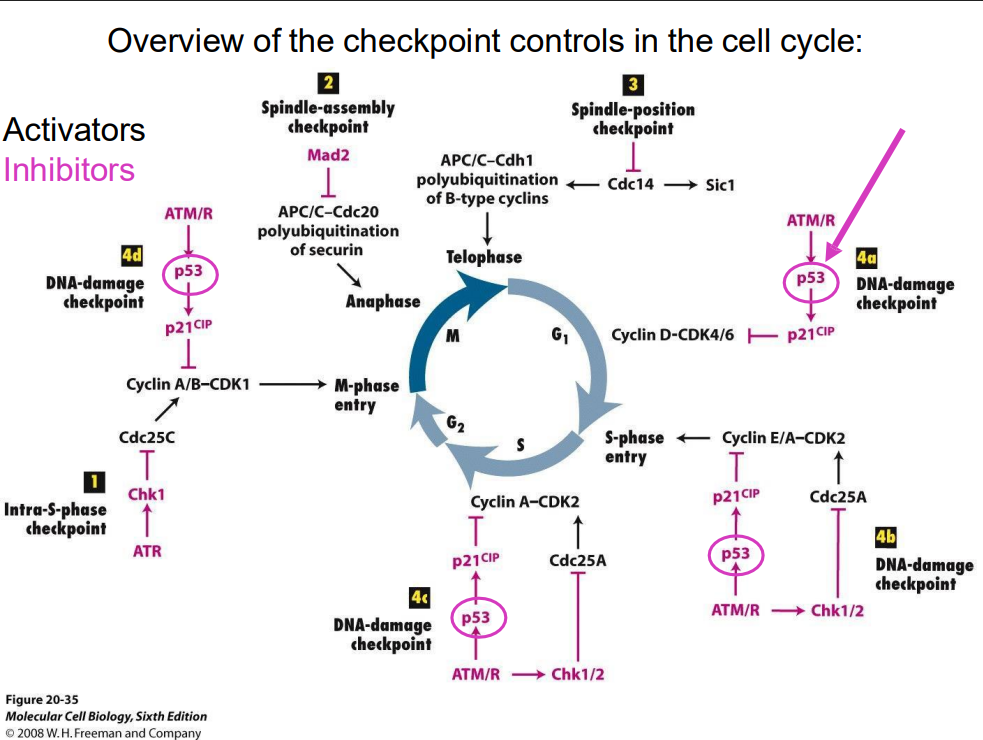
Checkpoints in cell cycle when biochemical processes are used to monitor "internal conditions" and stop if something is wrong
OBSERVATIONS:
1. unreplicated DNA -> prevents entry into M (S arrest)
2. Defects in assembly of mitotic spindle OR defects in attachment of kinetochores to microtubules -> stops degredation of anaphase inhibitor (M arrest)
3. DNA damage (UV irradiation etc.) -> cells will not pass G1 -> S (G1 arrest) OR G2 -> M (G2 arrest)
Overview of checkpoint controls in the cell cycle (no learning outcome)
DIAGRAM ON SLIDE 40 (WHOLE SLIDE)
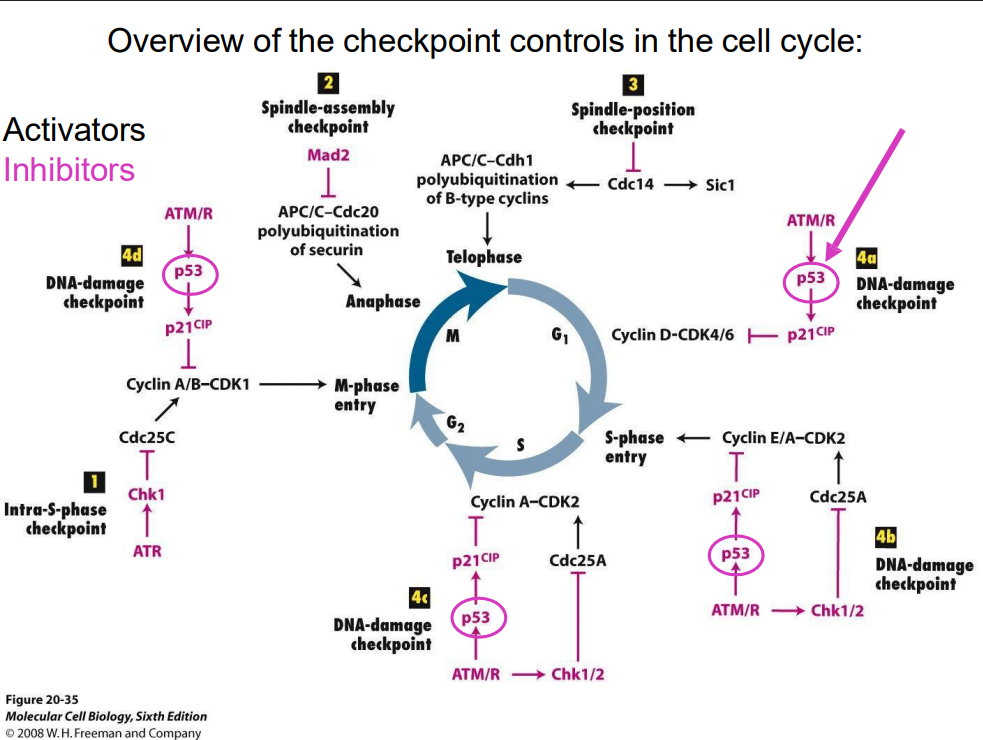
p53 is commonly referred to as
the guardian of the genome
- most frequently mutated gene in human canceres
- need to be intact to prevent bad
Ways to tell mutations
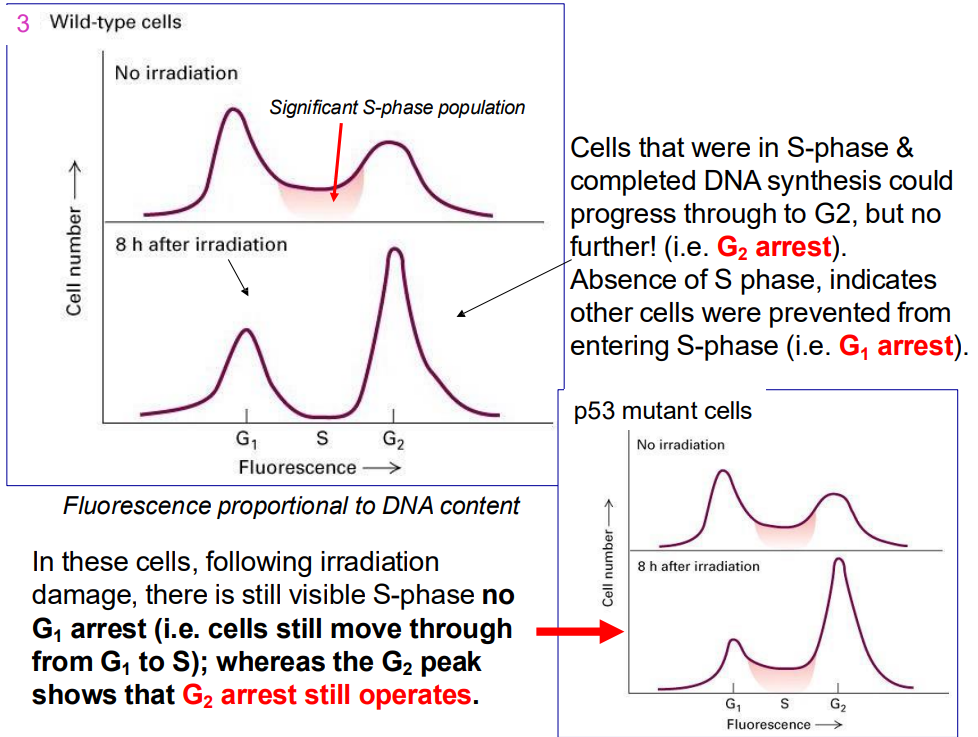
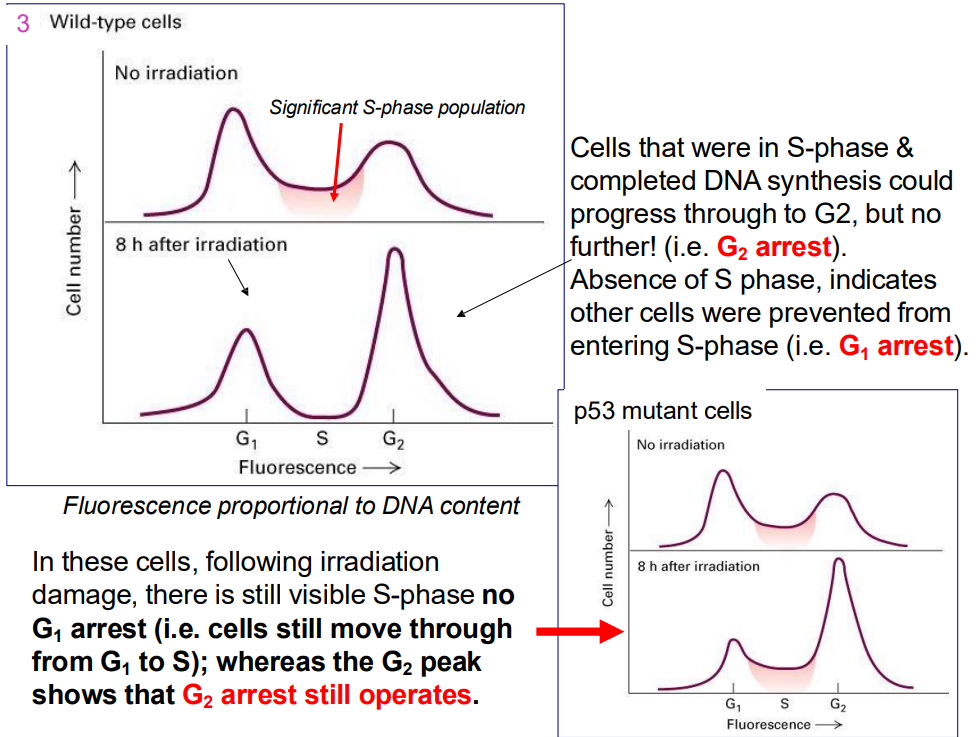
- Cells that were in S-phase & completed DNA synthesis could progress through to G2, but no further! (i.e. G2 arrest). Absence of S phase, indicates other cells were prevented from entering S-phase (i.e. G1 arrest).
.
- In these cells, following irradiation damage, there is still visible S-phase no G1 arrest (i.e. cells still move through from G1 to S); whereas the G2 peak shows that G2 arrest still operates.
.
- remember that p53 is the cruical guardian of genome that blocks progression if it senses any sort of dNA damage across all key checkpoints
p53 protein function = transcription factor, 53 kDA protein (thats where the name comes form, p for protein, 53)
- first identified as a tumour suppressor protein (mutant p53 found in around 50% human tumours)
- cells lacking functional p53 do NOT arrest in G1 (p53 also contributes to G2 arrest)
- p53 normally at low levels in cels (i.e it is unstable)
.
DAMAGED DNA (indirectly) stabilises p53 (levels rise)
- p53 then able to increase in concentration, binds to its specific response elements, and then upregulates expression of specific proteins
.
The main function of p53 is to act as a tumor suppressor by protecting the genome from damage through a variety of responses. It is often called the "guardian of the genome" because it is activated by cellular stress, such as DNA damage, and can either pause the cell cycle for DNA repair or trigger cell death (apoptosis) if the damage is too severe. This prevents cells with damaged DNA from becoming cancerous.
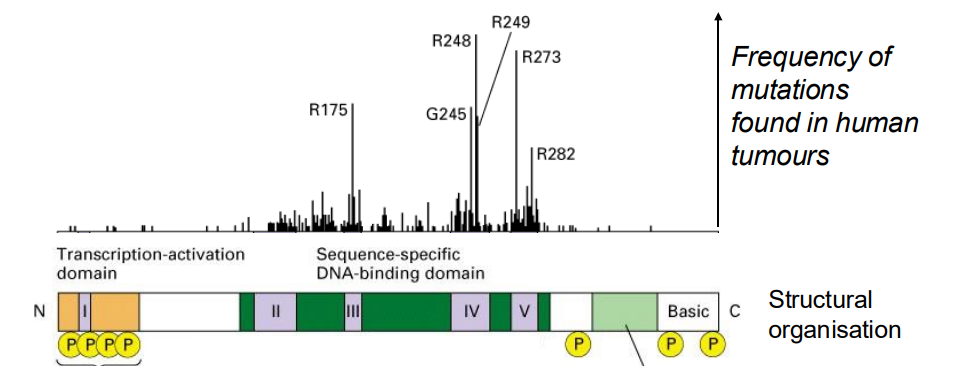
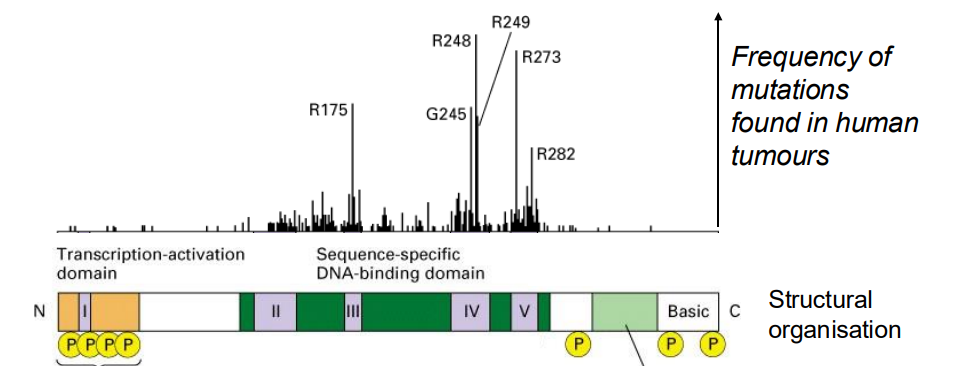
p53 protein is a transcription factor
- over 50% of all tumours have mutations in p53
.
- almost all mutations abolish its ability to bind DNA and activate specific gene transcription
- What are the target genes of p53?
.
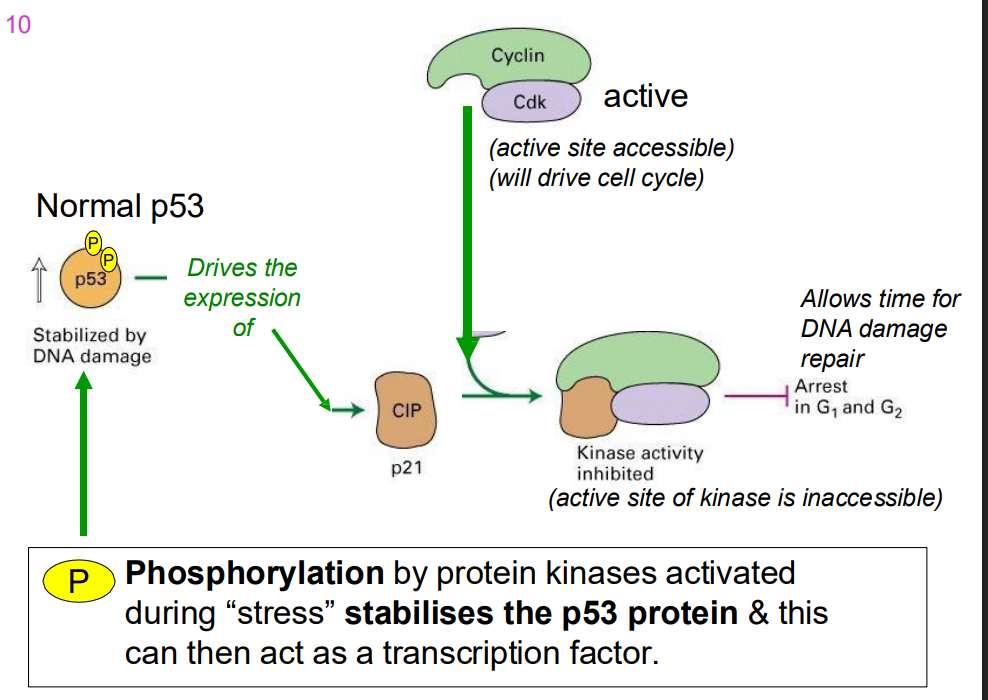
- one important gene encodes the cyclin kinase inhibitor (CIP) p21 (21 kDA), an inhibitor of G1 cyclin-dependent kinases
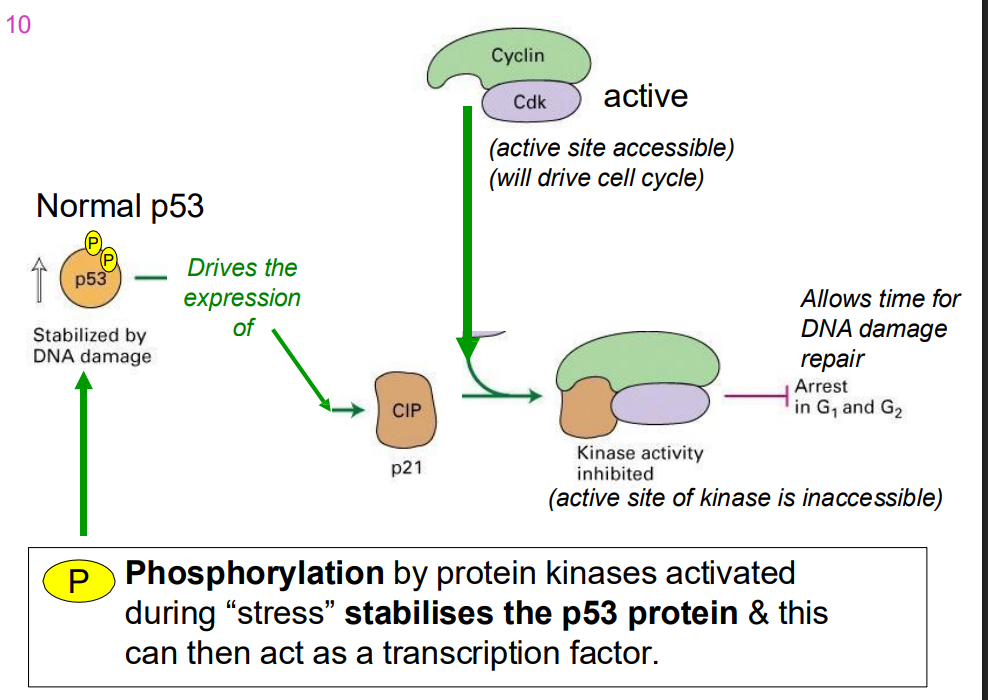
p53 and CIP
- phosphorylation by protein kinases activated during "stress" stabilises the p53 protein and this can then act as a trasncfritopn factor
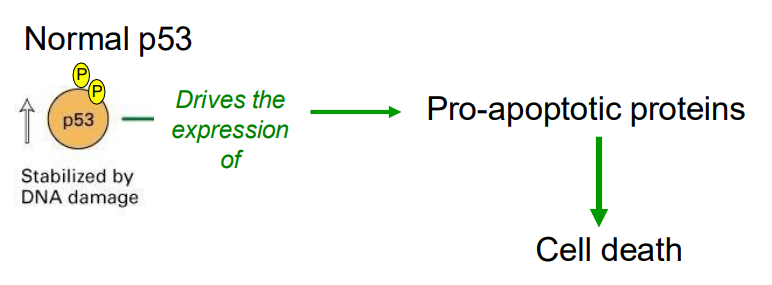
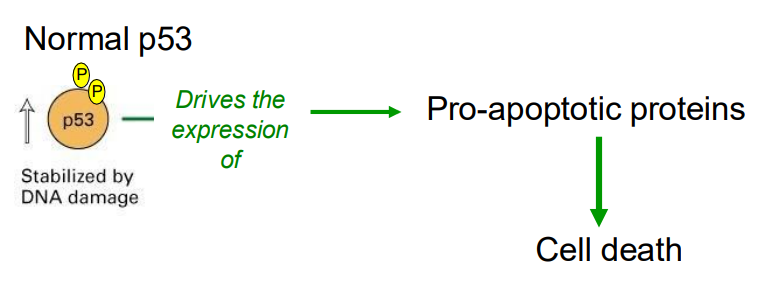
When DNA damage is extensive
- p53 drives the expression of pro-apoptotic rptoeins, whjcih leads to cell death
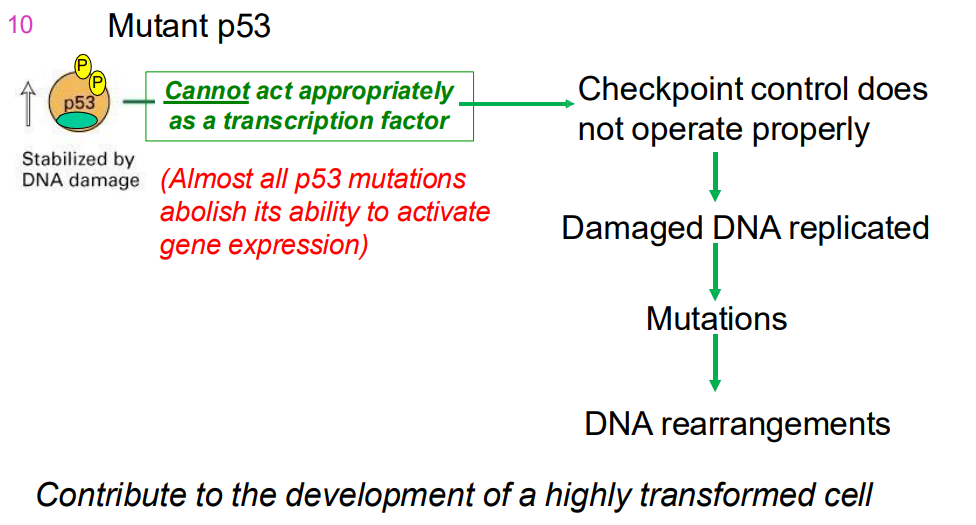
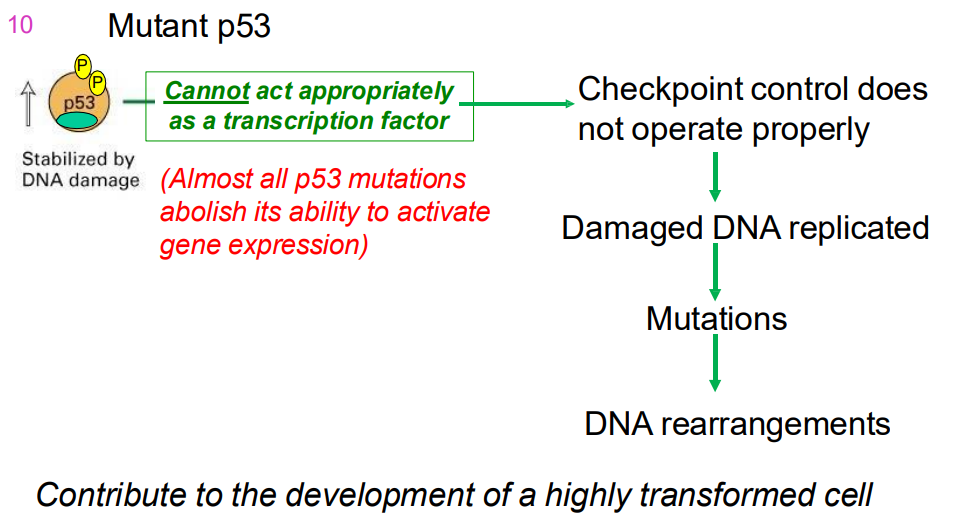
MUTANT p53
- cannot act as a transcriptoin factor properly (almost all p53 mutations abolish its ability to activtate gene expresion)
- checkpoint control does not operate properly
- dmaaged DNA replication
- mutations
- DNA rearrangemnts
.
- contributes to development of highly transofmred cell
- Cells with mutations in both p53 alleles (genes from both parents) do not show delayed entry into S phase & do not apoptose → tumour formation.
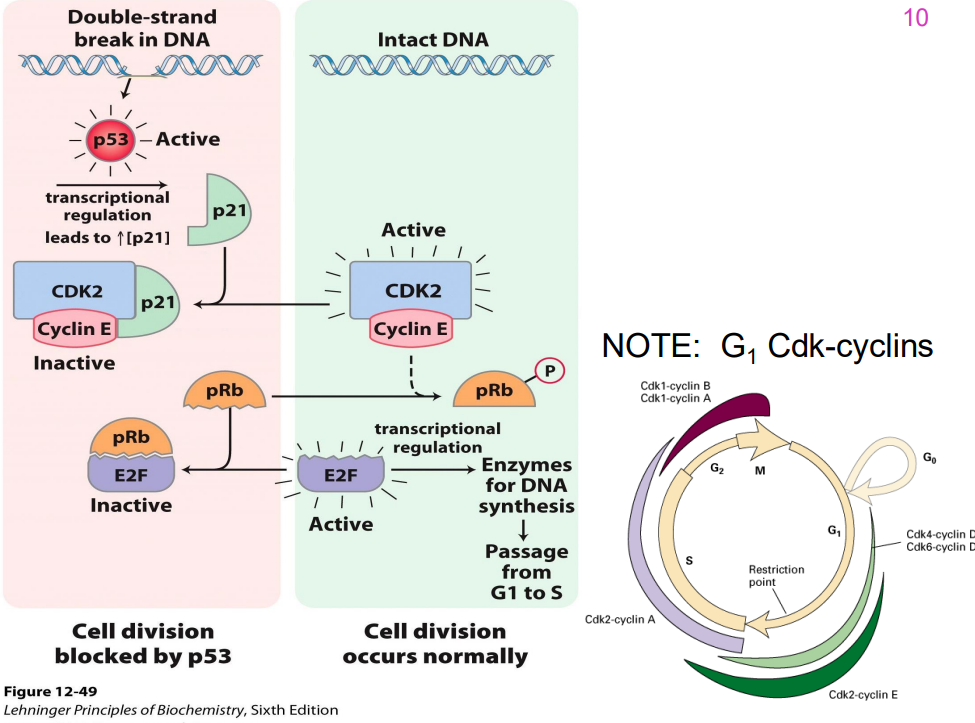
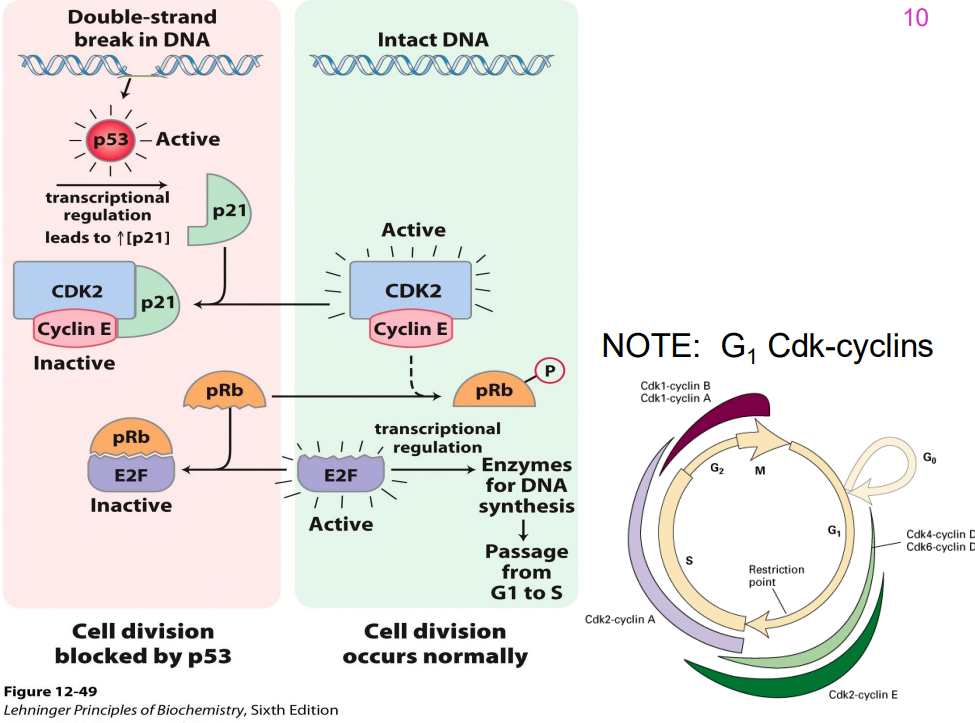
IMPORTANT DIAGRAM
DIAGRAM ON SLIDE 48
Apoptosis (Programmed Cell death, PCD)
- sequential destruction of cell (fragmentation of chromosomes, organelle disruption fragmentation of cell)
.
DRIVEN BY CASPASES:
- cysteine-containing aspartate-specific proteases normally inactive as zymogen (harmless to cell)
- zymogen (an inactive substance which is converted into an enzyme when activated by another enzyme) form activated by proteolysis
- active caspases target other proteins for destruction
.
- basically they contain cysteine and they target aspartate residues when breaking things down
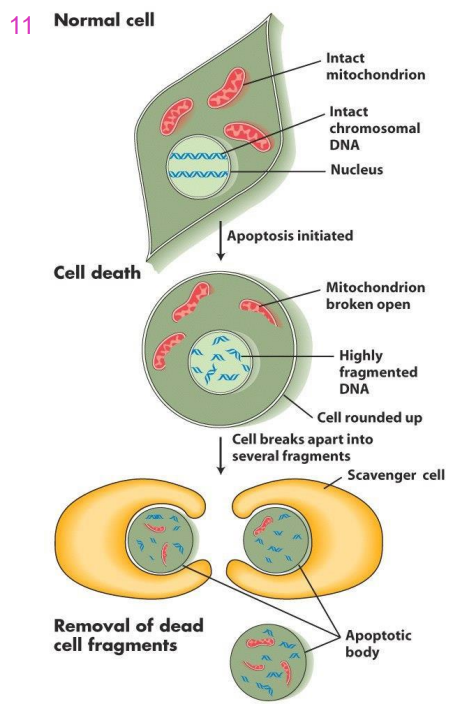
Events in Apoptosisq
- first the DNA of the chromosomes is fragmented, organelle structure is disrupted and cell loses its shape
- The cell breaks up into small fragments called apoptotic bodies
- Phagocytosed (eaten up) by scavenger cells
Caspases
- cysteine-containing aspartate specific proteases
- Proteases = proteins cleave proteins, process proteolysis
- when activated caspase cleave target proteins at aspartate residues. These target proteins initiate fragmentation of DNA, organelle disruption etc.
- in healthy cells, caspases are in an inactive state called zymogen -> inactive precursor -> must be cleaved for activation
TWO CLASSES OF CASPASES
Initiators and Executioners
Initiator Caspase and Executioner Caspase
- cleaved in response to activation signals. They in turn cleave one of the executioner caspases, which in turn cleaves another until all active
- Apoptosis (PCD) is mediated by a sequential cascade of proteolytic events that activate enzymes that destroy several key targeted cellular components
- Allows for a rapid response to a pro-apoptotic signals
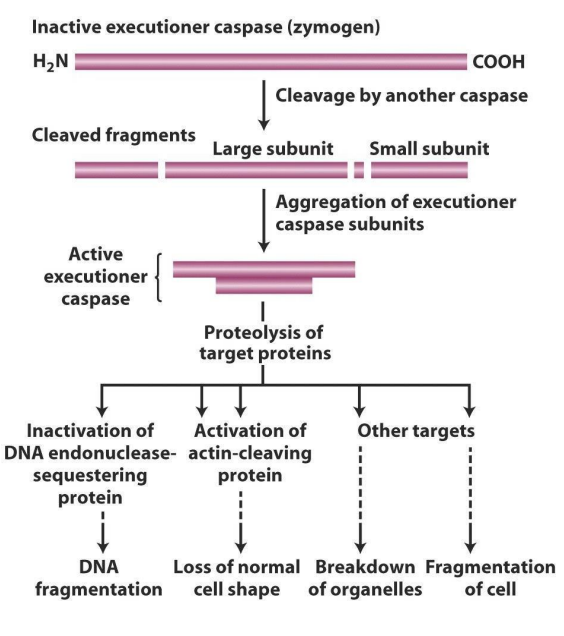
How do executioner caspases work?
- in addition to activating other caspases, executioner caspases cleave target proteins in the damaged cell
- one target is a "sequestering" protein that forms a complex with DNA endonuclease, holding it in the cytoplasm
- Sequestering protein cleaved, endonuclease free to enter nucleus, chop up DNA
- another target is protein, cleaved by caspases, cleaves actin, major component cytoskeleton and loss of cell shape
.
- it first targets DNA endonuclease-sequestering protein (which is a protein that sequesters binding DNA endonuclease, basically chops up DNA)
- actin gives cell shape
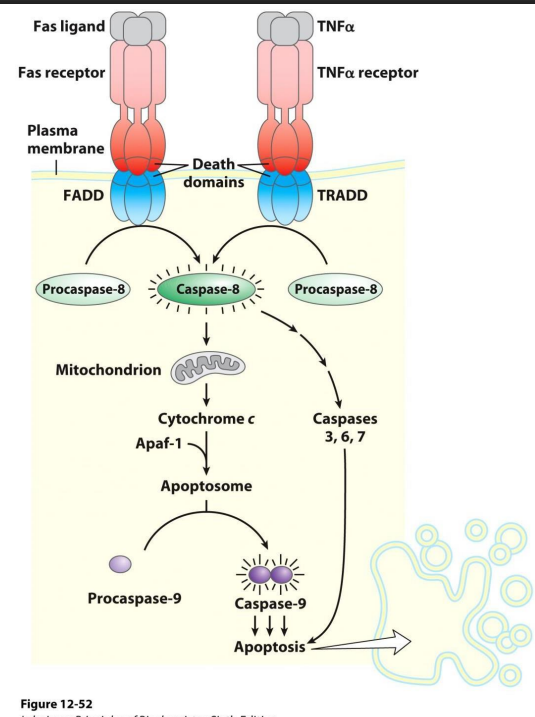
Apoptosis poristive controkls
NO LEANRING OUTOCME
Proliferation and Apoptosis Conbtrls NO LEARNING OUTCOME
INTRACELLULAR SINGAL,S
- cel cycle negative contorls: inhibition of Cdk-cyclin
- cell cycle positive ceontorls: activation of Cdk-cyclin
- apoptosis negative controls: maintain system "off"
- apoptosis positive controls: rapid response
.
INTERCELLULAR SIGNALS:
- based on cell-cell communication secreted molecules and direct cell to cell contact